Base acid salt water
Home » Science Education » Base acid salt waterBase acid salt water
Base Acid Salt Water. H aq cl aq na aq oh aq na aq cl aq h 2 o l writing the final ionic equation without spectator ions na and cl. Acid alkali salt water. You need to learn these two examples. Nitric acid sodium hydroxide sodium nitrate.
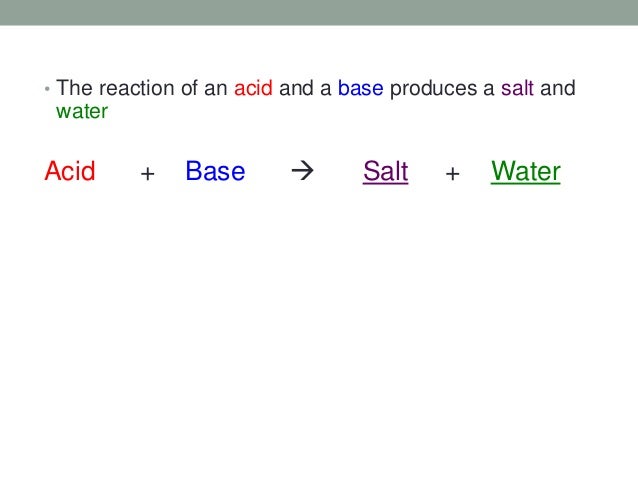 3 Acid Base Reactions From slideshare.net
3 Acid Base Reactions From slideshare.net
This means that these oxides have the properties of a base and acid. In this reaction the ion of h and oh will create water. You need to learn these two examples. An acid and base react to form a salt and water in a n reaction. The ion of non metal element of acid and ion of metal element of base will produce salt. When the acid and base react they will form the new substance named salt.
Acid metal hydroxide à salt water.
The products of this reaction are a salt and water. The correct formula for sulfuric acid is. Acid reactions with carbonates and hydrogencarbonates acids react with metal carbonates and hydrogencarbonates in the same way. Metal oxides and hydroxides are bases. Hydrochloric acid sodium hydroxide à sodium chloride water. The products of this reaction are a salt and water.
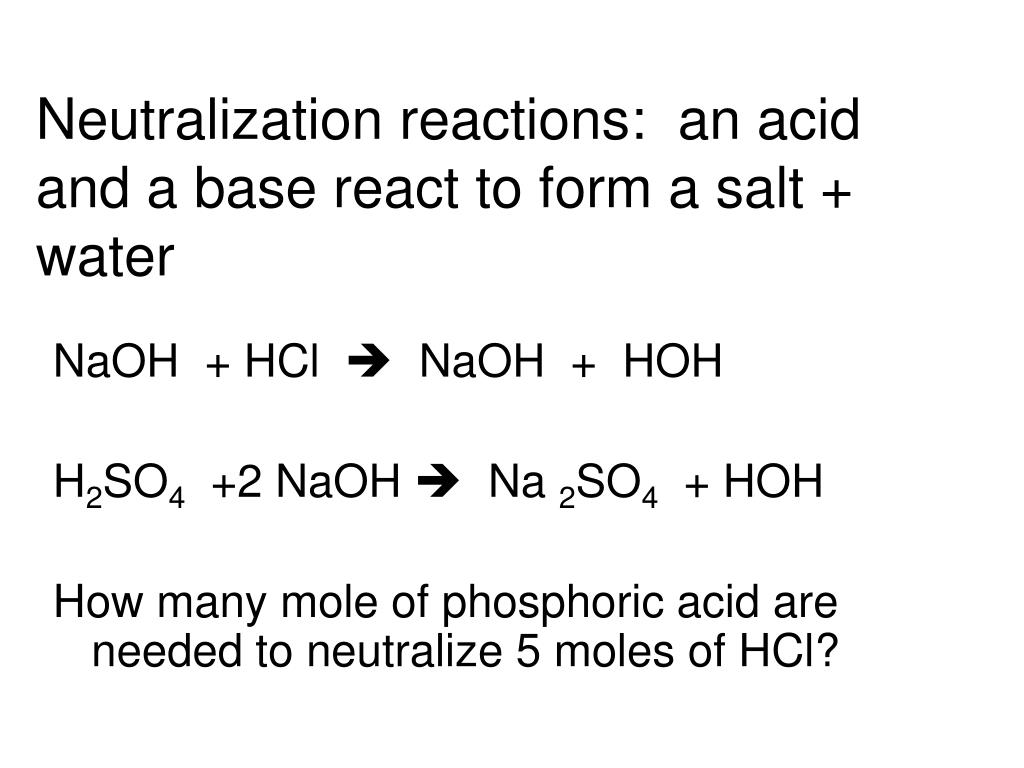 Source: slideserve.com
Source: slideserve.com
Acid metal hydroxide à salt water. A salt and water are produced when acids react with alkalis. Hcl aq naoh aq nacl aq h 2 o l writing ionic equation. Acid base salt water. The ion of non metal element of acid and ion of metal element of base will produce salt.
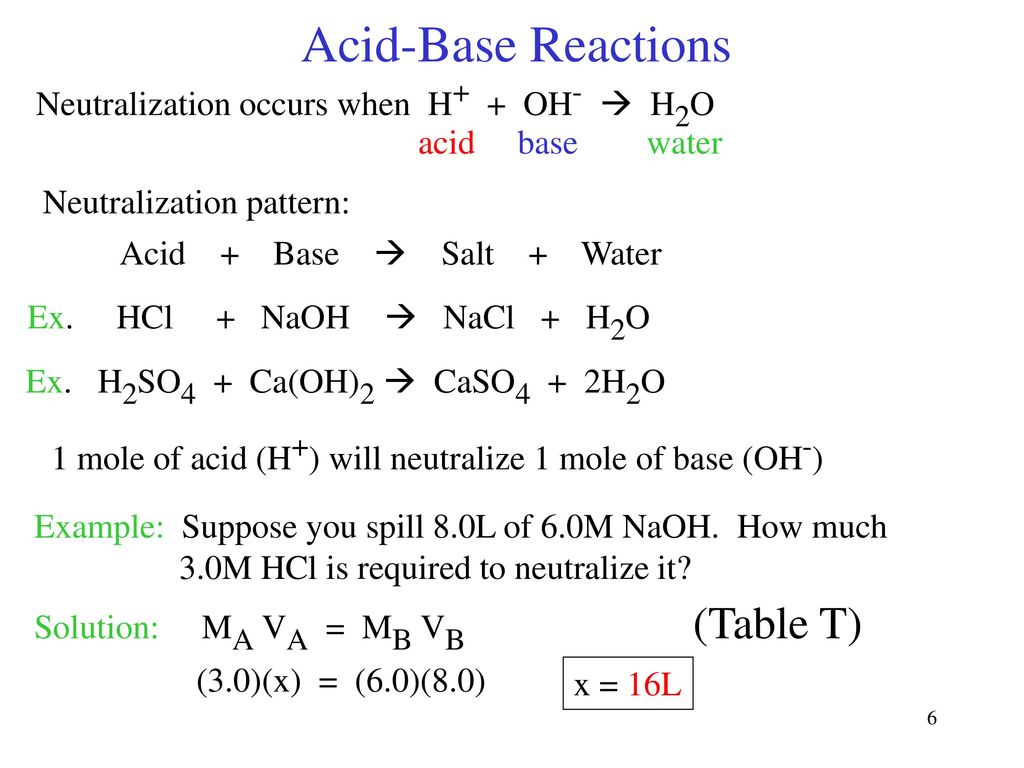 Source: slideplayer.com
Source: slideplayer.com
Metal oxides and hydroxides are bases. Acid metal hydroxide à salt water. Metal oxides and hydroxides are bases. Acid reactions with carbonates and hydrogencarbonates acids react with metal carbonates and hydrogencarbonates in the same way. Acid base salt water since the reaction between acid and base both neutralize each other hence it is also known as neutralization reaction.
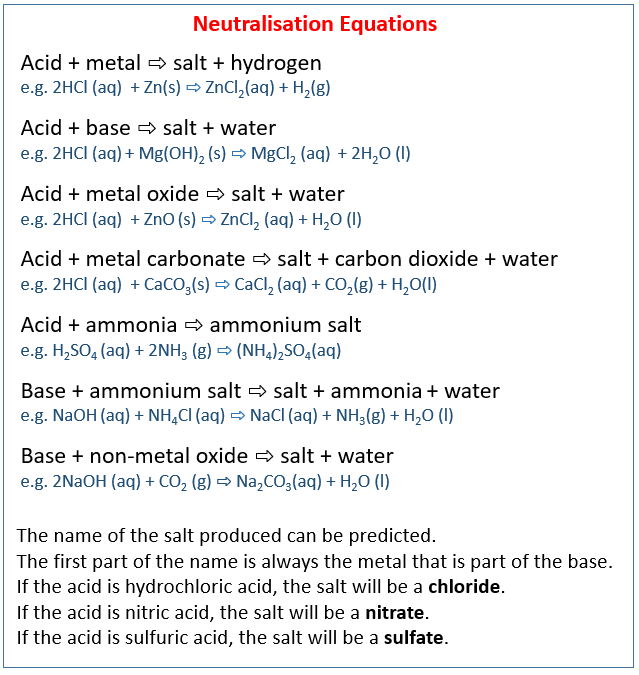 Source: onlinemathlearning.com
Source: onlinemathlearning.com
You need to learn these two examples. When the acid and base react they will form the new substance named salt. Acid alkali salt water. Examples are zinc oxide hydroxide and aluminium oxide hydroxide. Sodium chloride and water are formed when hydrochloric acid reacts with sodium hydroxide a strong base.
 Source: slideshare.net
Source: slideshare.net
This means that these oxides have the properties of a base and acid. Every base reacts with an acid to produce salt and water. Amphoteric oxides react with either a base or acid to form salt and water. The products of this reaction are a salt and water. Metal oxides and hydroxides are bases.
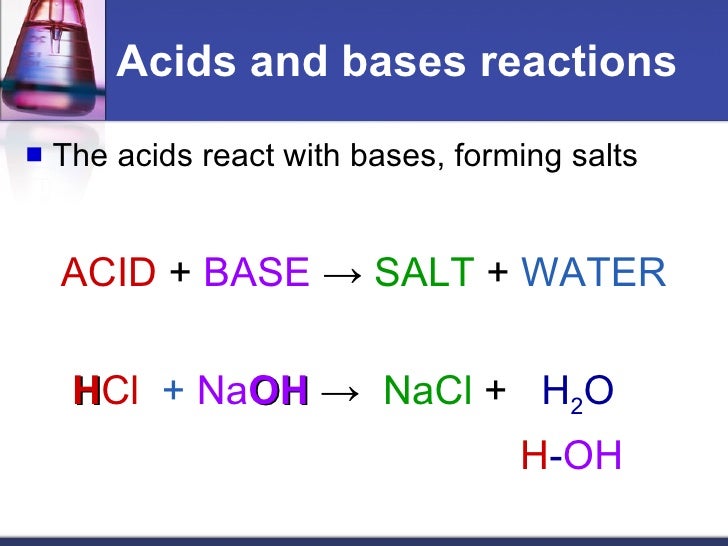 Source: slideshare.net
Source: slideshare.net
Every base reacts with an acid to produce salt and water. The correct formula for sulfuric acid is. Acid base salt water. Identify the bronsted lowry acid in the following reaction. Acid base salt water since the reaction between acid and base both neutralize each other hence it is also known as neutralization reaction.
 Source: slideplayer.com
Source: slideplayer.com
The reaction of an acid with a base is called a neutralization reaction. Acid reactions with carbonates and hydrogencarbonates acids react with metal carbonates and hydrogencarbonates in the same way. The reaction of an acid with a base is called a neutralization reaction. The word equation for this reaction is. Examples are zinc oxide hydroxide and aluminium oxide hydroxide.
Source: physicscatalyst.com
Hydrochloric acid sodium hydroxide à sodium chloride water. Acid alkali salt water. In this reaction the ion of h and oh will create water. The ion of non metal element of acid and ion of metal element of base will produce salt. The reaction of an acid with a base is called a neutralization reaction.
 Source: slideplayer.com
Source: slideplayer.com
The products of this reaction are a salt and water. Identify the bronsted lowry acid in the following reaction. Acid metal hydroxide à salt water. Nitric acid sodium hydroxide sodium nitrate. Amphoteric oxides react with either a base or acid to form salt and water.
 Source: 2classnotes.com
Source: 2classnotes.com
The reaction of an acid with a base is called a neutralization reaction. Hcl aq naoh aq nacl aq h 2 o l writing ionic equation. The reaction of an acid with a base is called a neutralization reaction. Nitric acid sodium hydroxide sodium nitrate. The name of al oh 3 is.
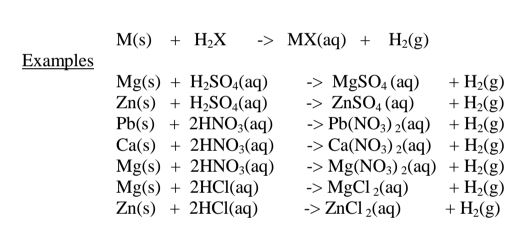 Source: advance-africa.com
Source: advance-africa.com
Acid alkali salt water. In this traditional representation an acid base neutralization reaction is formulated as a double replacement reaction. This means that these oxides have the properties of a base and acid. An acid and base react to form a salt and water in a n reaction. Hcl aq naoh aq nacl aq h 2 o l writing ionic equation.
Source: quora.com
Alkalis are soluble bases. Acid base salt water. These reactions produce salt water and carbon dioxide. The reaction of an acid with a base is called a neutralization reaction. The ion of non metal element of acid and ion of metal element of base will produce salt.
 Source: slideserve.com
Source: slideserve.com
Acid base salt water. The reaction of an acid with a base is called a neutralization reaction. Acid reactions with carbonates and hydrogencarbonates acids react with metal carbonates and hydrogencarbonates in the same way. In this reaction the ion of h and oh will create water. Acid metal hydroxide à salt water.
 Source: bright-culture.com
Source: bright-culture.com
Nitric acid sodium hydroxide sodium nitrate. This means that these oxides have the properties of a base and acid. The reaction of an acid with a base is called a neutralization reaction. Acid metal hydroxide à salt water. Sodium chloride and water are formed when hydrochloric acid reacts with sodium hydroxide a strong base.
 Source: goodscience.com.au
Source: goodscience.com.au
The correct formula for sulfuric acid is. This means that these oxides have the properties of a base and acid. Amphoteric oxides react with either a base or acid to form salt and water. Acid base salt water. Identify the bronsted lowry acid in the following reaction.
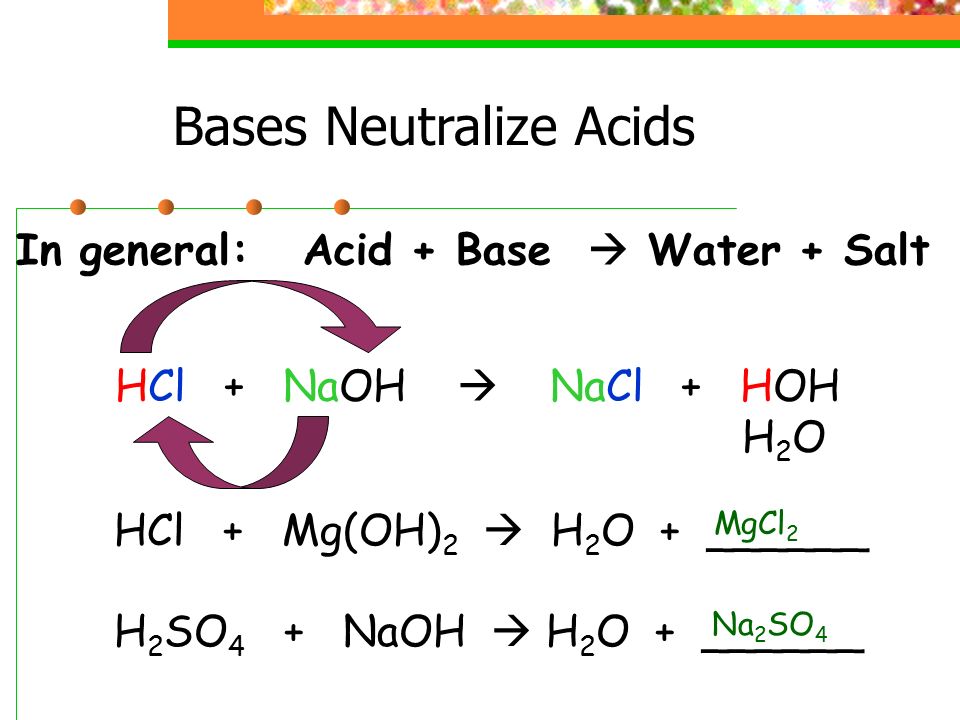 Source: sciencewithmrsb.weebly.com
Source: sciencewithmrsb.weebly.com
A salt and water are produced when acids react with alkalis. Nitric acid sodium hydroxide sodium nitrate. H aq cl aq na aq oh aq na aq cl aq h 2 o l writing the final ionic equation without spectator ions na and cl. In this traditional representation an acid base neutralization reaction is formulated as a double replacement reaction. Acid base salt water.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title base acid salt water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.
