Calcium hydroxide preparation
Home » Science Education » Calcium hydroxide preparationCalcium hydroxide preparation
Calcium Hydroxide Preparation. Cao h 2 o ca oh 2. The temperature of this reaction is more than enough to boil water and can produce a caustic spray of limewater that can seriously injure an overenthusiastic chemist so the addition must be slow and the temperature must be watched. Calcium hydroxide is usually prepared by the highly exothermic addition of calcium oxide to water in a process called slaking. It forms a fluffy charged solid that aids in the removal of smaller particles from water resulting in a clearer product.
 Preparation Of Zinc And Calcium Hydroxide Modified Fly Ash And Its Utilization As Filler In Nylon 6 Semantic Scholar From semanticscholar.org
Preparation Of Zinc And Calcium Hydroxide Modified Fly Ash And Its Utilization As Filler In Nylon 6 Semantic Scholar From semanticscholar.org
Generally calcium hydroxide is prepared by mixing water and calcium oxide also known as quick lime. Cao h 2 o ca oh 2. It is a white powder. The aqueous solution is known as lime water and a suspension of slaked lime in water is known as milk of lime. The structure of a ca oh 2 molecule is illustrated below. Calcium hydroxide is prepared by adding water to quick lime cao.
Calcium hydroxide is prepared by adding water to quick lime cao.
When carbon dioxide is passed through lime water it turns milky. Generally calcium hydroxide is prepared by mixing water and calcium oxide also known as quick lime. Calcium hydroxide is prepared by adding water to quick lime cao. It forms a fluffy charged solid that aids in the removal of smaller particles from water resulting in a clearer product. It is sparingly soluble in water. The aqueous solution is known as lime water and a suspension of slaked lime in water is known as milk of lime.

It is also prepared on smaller scales by the reaction between aqueous calcium chloride and sodium hydroxide. One significant application of calcium hydroxide is as a flocculant in water and sewage treatment. Generally calcium hydroxide is prepared by mixing water and calcium oxide also known as quick lime. The structure of a ca oh 2 molecule is illustrated below. When carbon dioxide is passed through lime water it turns milky.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The temperature of this reaction is more than enough to boil water and can produce a caustic spray of limewater that can seriously injure an overenthusiastic chemist so the addition must be slow and the temperature must be watched. It is sparingly soluble in water. Preparation of calcium hydroxide. The chemical reaction between sodium hydroxide and calcium chloride dissolved in water aqueous cacl 2 also yields this compound. The aqueous solution is known as lime water and a suspension of slaked lime in water is known as milk of lime.
 Source: igcse-chemistry-edexcel.blogspot.com
Source: igcse-chemistry-edexcel.blogspot.com
The aqueous solution is known as lime water and a suspension of slaked lime in water is known as milk of lime. It is a white powder. The chemical reaction between sodium hydroxide and calcium chloride dissolved in water aqueous cacl 2 also yields this compound. The aqueous solution is known as lime water and a suspension of slaked lime in water is known as milk of lime. Calcium hydroxide is produced commercially by reacting calcium oxide lime or quicklime with water.
 Source: semanticscholar.org
Source: semanticscholar.org
The aqueous solution is known as lime water and a suspension of slaked lime in water is known as milk of lime. When carbon dioxide is passed through lime water it turns milky. The temperature of this reaction is more than enough to boil water and can produce a caustic spray of limewater that can seriously injure an overenthusiastic chemist so the addition must be slow and the temperature must be watched. Calcium hydroxide is produced commercially by reacting calcium oxide lime or quicklime with water. Calcium hydroxide is usually prepared by the highly exothermic addition of calcium oxide to water in a process called slaking.
 Source: dline.ee
Source: dline.ee
When carbon dioxide is passed through lime water it turns milky. One significant application of calcium hydroxide is as a flocculant in water and sewage treatment. Cao h 2 o ca oh 2. The structure of a ca oh 2 molecule is illustrated below. Calcium hydroxide is prepared by adding water to quick lime cao.
 Source: freechemistryonline.com
Source: freechemistryonline.com
When carbon dioxide is passed through lime water it turns milky. It is a white powder. It is sparingly soluble in water. Calcium hydroxide is prepared by adding water to quick lime cao. It is also prepared on smaller scales by the reaction between aqueous calcium chloride and sodium hydroxide.
 Source: researchgate.net
Source: researchgate.net
Cao h 2 o ca oh 2. The structure of a ca oh 2 molecule is illustrated below. It is also prepared on smaller scales by the reaction between aqueous calcium chloride and sodium hydroxide. The chemical reaction between sodium hydroxide and calcium chloride dissolved in water aqueous cacl 2 also yields this compound. It forms a fluffy charged solid that aids in the removal of smaller particles from water resulting in a clearer product.
 Source: slideshare.net
Source: slideshare.net
The temperature of this reaction is more than enough to boil water and can produce a caustic spray of limewater that can seriously injure an overenthusiastic chemist so the addition must be slow and the temperature must be watched. It forms a fluffy charged solid that aids in the removal of smaller particles from water resulting in a clearer product. It is sparingly soluble in water. Generally calcium hydroxide is prepared by mixing water and calcium oxide also known as quick lime. The aqueous solution is known as lime water and a suspension of slaked lime in water is known as milk of lime.
 Source: studocu.com
Source: studocu.com
Calcium hydroxide is commonly used to prepare lime mortar. The aqueous solution is known as lime water and a suspension of slaked lime in water is known as milk of lime. Calcium hydroxide is commonly used to prepare lime mortar. Calcium hydroxide is usually prepared by the highly exothermic addition of calcium oxide to water in a process called slaking. Cao h 2 o ca oh 2.
 Source: sodimate-inc.com
Source: sodimate-inc.com
It is a white powder. Preparation of calcium hydroxide. The temperature of this reaction is more than enough to boil water and can produce a caustic spray of limewater that can seriously injure an overenthusiastic chemist so the addition must be slow and the temperature must be watched. The aqueous solution is known as lime water and a suspension of slaked lime in water is known as milk of lime. Calcium hydroxide is usually prepared by the highly exothermic addition of calcium oxide to water in a process called slaking.
 Source: youtube.com
Source: youtube.com
Preparation of calcium hydroxide. It forms a fluffy charged solid that aids in the removal of smaller particles from water resulting in a clearer product. One significant application of calcium hydroxide is as a flocculant in water and sewage treatment. It is sparingly soluble in water. It is also prepared on smaller scales by the reaction between aqueous calcium chloride and sodium hydroxide.
 Source: youtube.com
Source: youtube.com
Generally calcium hydroxide is prepared by mixing water and calcium oxide also known as quick lime. It forms a fluffy charged solid that aids in the removal of smaller particles from water resulting in a clearer product. One significant application of calcium hydroxide is as a flocculant in water and sewage treatment. It is also prepared on smaller scales by the reaction between aqueous calcium chloride and sodium hydroxide. Generally calcium hydroxide is prepared by mixing water and calcium oxide also known as quick lime.
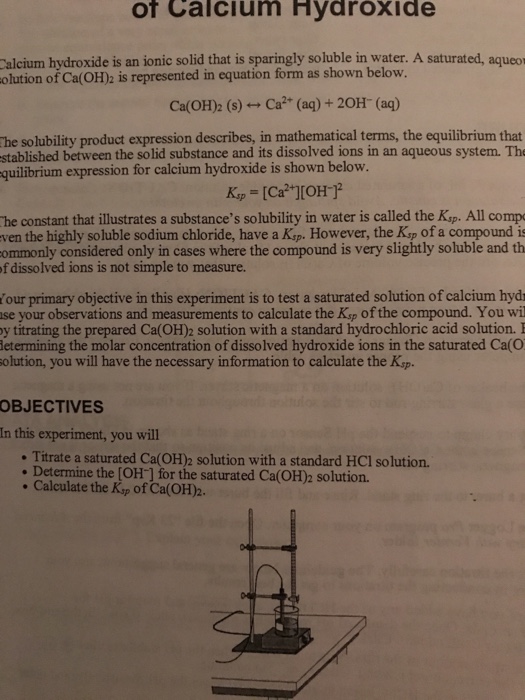
One significant application of calcium hydroxide is as a flocculant in water and sewage treatment. Cao h 2 o ca oh 2. Calcium hydroxide is produced commercially by reacting calcium oxide lime or quicklime with water. Generally calcium hydroxide is prepared by mixing water and calcium oxide also known as quick lime. Preparation of calcium hydroxide.
 Source: patentsencyclopedia.com
Source: patentsencyclopedia.com
Preparation of calcium hydroxide. Preparation of calcium hydroxide. It is also prepared on smaller scales by the reaction between aqueous calcium chloride and sodium hydroxide. One significant application of calcium hydroxide is as a flocculant in water and sewage treatment. Calcium hydroxide is commonly used to prepare lime mortar.
Source: researchgate.net
The temperature of this reaction is more than enough to boil water and can produce a caustic spray of limewater that can seriously injure an overenthusiastic chemist so the addition must be slow and the temperature must be watched. It is sparingly soluble in water. One significant application of calcium hydroxide is as a flocculant in water and sewage treatment. Calcium hydroxide is commonly used to prepare lime mortar. Calcium hydroxide is prepared by adding water to quick lime cao.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title calcium hydroxide preparation by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.
