Calculating moles in a solution
Home » Science Education » Calculating moles in a solutionCalculating moles in a solution
Calculating Moles In A Solution. If you know the moles of solute in a solution and you know the volume of the solution you can calculate the concentration of the solution in mol l 1using c n v. This tremendous value refers to avogadro s number. Mole fraction or molar fraction is the number of moles of one component of a solution divided by the total number of moles of all chemical species. If you wanted to find the concentration of the hydrochloric acid you could use our concentration calculator.
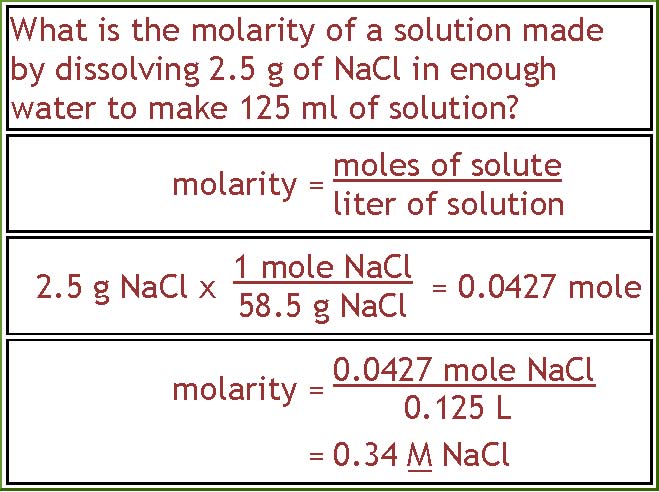 Calculations Using Molarity From people.tamu.edu
Calculations Using Molarity From people.tamu.edu
Note that moles cancel out when calculating mole fraction so it is a unitless value. C is the molar concentration in mol l molar or m. Solve the equation and label the answer m. To know how to calculate moles the equation is. A mole calculation in solution requires using the molarity formula. This tremendous value refers to avogadro s number.
By rearranging the molarity formula where molarity equals moles of solute divided by liters of solution the amount of moles may be calculated.
The following diagram shows the conversion between mole and mass. Mass number of moles molar mass. To know how to calculate moles the equation is. The mole is the si unit of the measurement for the amount of a substance. In one mole of matter there is precisely 6 02214085774 10 using scientific notation atoms molecules or anything else. In this example m 0 45 mol 0 4 l 1 125 m.
 Source: wikihow.com
Source: wikihow.com
In this example m 0 45 mol 0 4 l 1 125 m. Divide the moles of solute by the volume of the solution in liters. Note some people express mole fraction as a percent not common. Molar solution concentration equation. The sum of all mole fractions adds up to 1.
 Source: youtube.com
Source: youtube.com
Molarity is defined as the number of moles of solute dissolved per liter of solution mol l m. Molarity is defined as the number of moles of solute dissolved per liter of solution mol l m. This is also referred to as molarity which is the most common method of expressing the concentration of a solute in a solution. How to calculate the number of moles of a substance when we are given the mass mass to mole conversion. To know how to calculate moles the equation is.
 Source: people.tamu.edu
Source: people.tamu.edu
C is the molar concentration in mol l molar or m. The volume of the solution and the solution concentration is needed. To know how to calculate moles the equation is. This is also referred to as molarity which is the most common method of expressing the concentration of a solute in a solution. This is where moles come in handy.
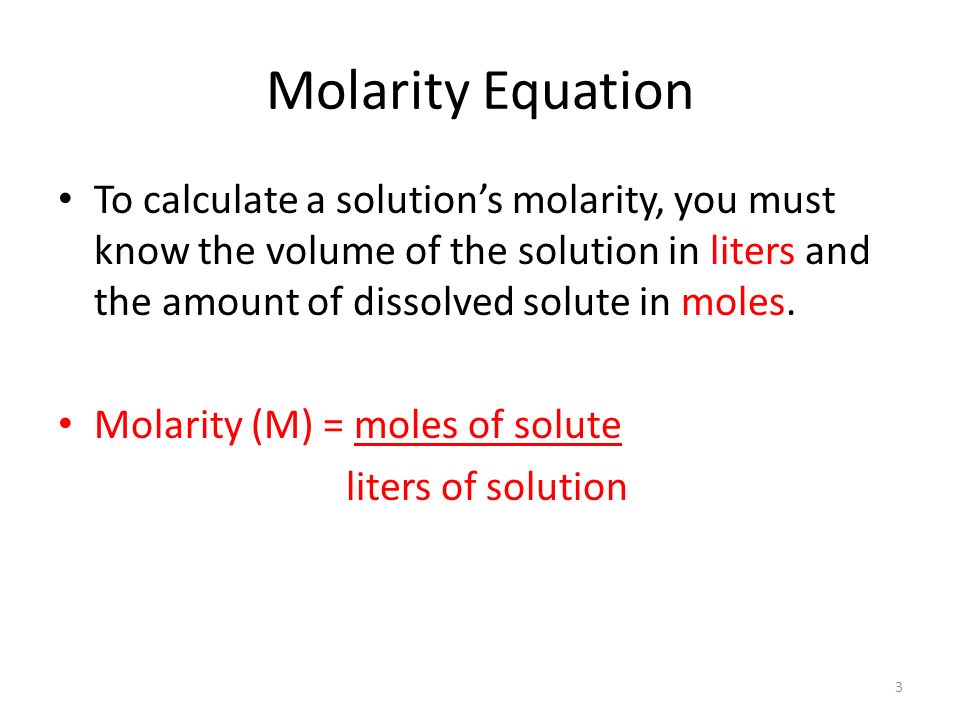 Source: socratic.org
Source: socratic.org
This tremendous value refers to avogadro s number. If you know the moles of solute in a solution and you know the volume of the solution you can calculate the concentration of the solution in mol l 1using c n v. Mass number of moles molar mass. This is where moles come in handy. To know how to calculate moles the equation is.
 Source: study.com
Source: study.com
Molarity is defined as the number of moles of solute dissolved per liter of solution mol l m. To know how to calculate moles the equation is. Mass number of moles molar mass. The following diagram shows the conversion between mole and mass. A mole calculation in solution requires using the molarity formula.
 Source: thoughtco.com
Source: thoughtco.com
Divide the moles of solute by the volume of the solution in liters. Note some people express mole fraction as a percent not common. How to calculate the number of moles of a substance when we are given the mass mass to mole conversion. The following diagram shows the conversion between mole and mass. Scroll down the page for more examples and solutions.
 Source: slideplayer.com
Source: slideplayer.com
It is therefore useful to find out exactly how many molecules of hcl are in the solution. To calculate the mass of a higher number of moles or even calculate the number of moles in a certain mass a formula triangle can be used. Using the formula triangle is straightforward. Set up your equation so the molarity m mol v where mol is the number of moles of the solute and v is the volume of the solvent. Mole fraction or molar fraction is the number of moles of one component of a solution divided by the total number of moles of all chemical species.
 Source: chem.fsu.edu
Source: chem.fsu.edu
Mole mass molecular weight. In one mole of matter there is precisely 6 02214085774 10 using scientific notation atoms molecules or anything else. If you know the moles of solute in a solution and you know the volume of the solution you can calculate the concentration of the solution in mol l 1using c n v. This is where moles come in handy. In this example m 0 45 mol 0 4 l 1 125 m.
 Source: researchgate.net
Source: researchgate.net
Using the formula triangle is straightforward. Note some people express mole fraction as a percent not common. The volume of the solution and the solution concentration is needed. If you know the moles of solute in a solution and you know the volume of the solution you can calculate the concentration of the solution in mol l 1using c n v. To calculate the mass of a higher number of moles or even calculate the number of moles in a certain mass a formula triangle can be used.
 Source: khanacademy.org
Source: khanacademy.org
Mole fraction or molar fraction is the number of moles of one component of a solution divided by the total number of moles of all chemical species. In one mole of matter there is precisely 6 02214085774 10 using scientific notation atoms molecules or anything else. It is therefore useful to find out exactly how many molecules of hcl are in the solution. To know how to calculate moles the equation is. Molar solution concentration equation.
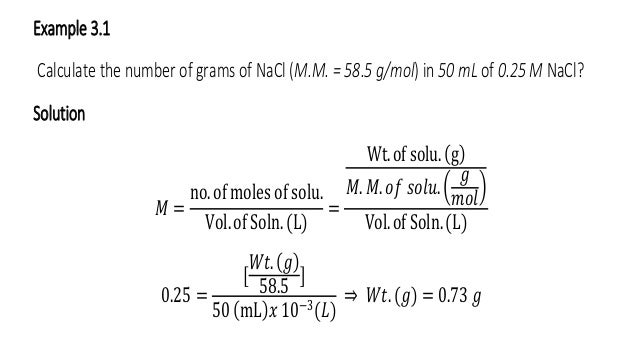 Source: slideshare.net
Source: slideshare.net
Scroll down the page for more examples and solutions. Set up your equation so the molarity m mol v where mol is the number of moles of the solute and v is the volume of the solvent. Mass number of moles molar mass. To calculate the mass of a higher number of moles or even calculate the number of moles in a certain mass a formula triangle can be used. This is where moles come in handy.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
The following diagram shows the conversion between mole and mass. Molar solution concentration equation. Molarity is defined as the number of moles of solute dissolved per liter of solution mol l m. Solve the equation and label the answer m. This is also referred to as molarity which is the most common method of expressing the concentration of a solute in a solution.
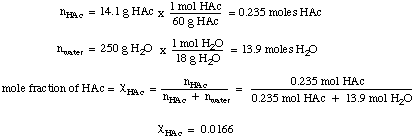 Source: topperlearning.com
Source: topperlearning.com
Mole fraction or molar fraction is the number of moles of one component of a solution divided by the total number of moles of all chemical species. Mass number of moles molar mass. Divide the moles of solute by the volume of the solution in liters. Note some people express mole fraction as a percent not common. Solve the equation and label the answer m.
 Source: slideplayer.com
Source: slideplayer.com
Molarity is defined as the number of moles of solute dissolved per liter of solution mol l m. This is also referred to as molarity which is the most common method of expressing the concentration of a solute in a solution. Mole mass molecular weight. To know how to calculate moles the equation is. In other words it s the unit of quantity similarly as a dozen or a gross.
 Source: nagwa.com
Source: nagwa.com
This is where moles come in handy. This is also referred to as molarity which is the most common method of expressing the concentration of a solute in a solution. If you know the moles of solute in a solution and you know the volume of the solution you can calculate the concentration of the solution in mol l 1using c n v. Mole mass molecular weight. The sum of all mole fractions adds up to 1.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title calculating moles in a solution by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.
