Charles and boyles law
Home » Science Education » Charles and boyles lawCharles and boyles law
Charles And Boyles Law. You can express this mathematically as. If the absolute temperature of the gas increases the gas volume also increases on the contrary if the absolute temperature of the gas decreases the gas volume also decreases. The two terms involved in charles law are directly proportional to each other while the terms involved in boyle s law are inversely proportional. If you increase the pressure 10 times the volume will decrease 10 times.
 More Boyle S Law And Charles Law Worksheet From yumpu.com
More Boyle S Law And Charles Law Worksheet From yumpu.com
That means that for example if you double the pressure you will halve the volume. Boyle s law charles s law and gay lussac s law form the combined gas law. This relationship is known as charles s law. If the absolute temperature of the gas increases the gas volume also increases on the contrary if the absolute temperature of the gas decreases the gas volume also decreases. V α t p constant. If you increase the pressure 10 times the volume will decrease 10 times.
For a fixed mass of gas at constant temperature the volume is inversely proportional to the pressure.
Boyle showed that the volume of a sample of a gas is inversely proportional to its pressure boyle s law charles and gay lussac demonstrated that the volume of a gas is directly proportional to its temperature in kelvins at constant pressure charles s law and avogadro postulated that the volume of a gas is directly proportional to the number of moles of gas present avogadro s law. You can express this mathematically as. Boyle s law is often used as part of an explanation on how the breathing system works in the human body. V α t p constant. Charles law is defined for a system with a constant pressure while boyle s law is defined for a system with constant temperature. If the absolute temperature of the gas increases the gas volume also increases on the contrary if the absolute temperature of the gas decreases the gas volume also decreases.
 Source: pinterest.com
Source: pinterest.com
That means that for example if you double the pressure you will halve the volume. Boyle s law charles s law and gay lussac s law form the combined gas law. For a fixed mass of gas at constant temperature the volume is inversely proportional to the pressure. The two terms involved in charles law are directly proportional to each other while the terms involved in boyle s law are inversely proportional. V α t p constant.
 Source: youtube.com
Source: youtube.com
If you increase the pressure 10 times the volume will decrease 10 times. Boyle s law is often used as part of an explanation on how the breathing system works in the human body. Boyle showed that the volume of a sample of a gas is inversely proportional to its pressure boyle s law charles and gay lussac demonstrated that the volume of a gas is directly proportional to its temperature in kelvins at constant pressure charles s law and avogadro postulated that the volume of a gas is directly proportional to the number of moles of gas present avogadro s law. If you increase the pressure 10 times the volume will decrease 10 times. Charles law is defined for a system with a constant pressure while boyle s law is defined for a system with constant temperature.
 Source: unacademy.com
Source: unacademy.com
For a fixed mass of gas at constant temperature the volume is inversely proportional to the pressure. What is the difference between charles law and boyle s law. The three gas laws in combination with avogadro s law can be generalized by the ideal gas law. Charles law is defined for a system with a constant pressure while boyle s law is defined for a system with constant temperature. This commonly involves explaining how the lung volume may be increased or decreased and thereby cause a relatively lower or higher air pressure within them in keeping with boyle s law.
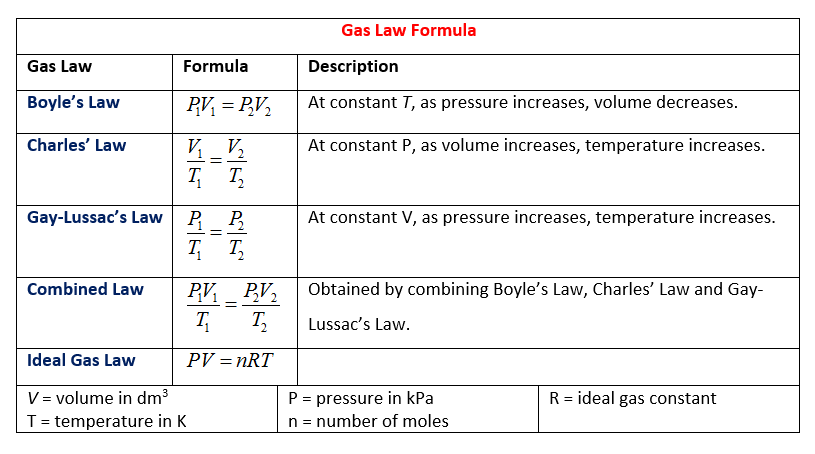 Source: onlinemathlearning.com
Source: onlinemathlearning.com
For a fixed mass of gas at constant temperature the volume is inversely proportional to the pressure. Boyle showed that the volume of a sample of a gas is inversely proportional to its pressure boyle s law charles and gay lussac demonstrated that the volume of a gas is directly proportional to its temperature in kelvins at constant pressure charles s law and avogadro postulated that the volume of a gas is directly proportional to the number of moles of gas present avogadro s law. Boyle s law is often used as part of an explanation on how the breathing system works in the human body. If you increase the pressure 10 times the volume will decrease 10 times. For a fixed mass of gas at constant temperature the volume is inversely proportional to the pressure.
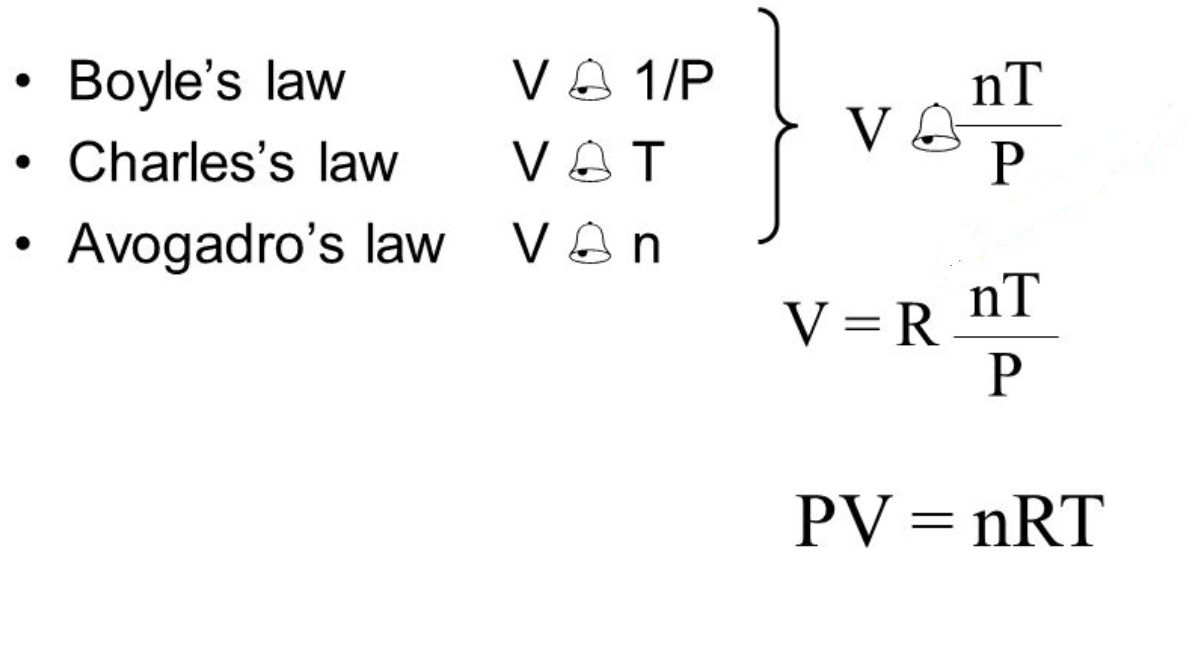 Source: chem.fsu.edu
Source: chem.fsu.edu
The three gas laws in combination with avogadro s law can be generalized by the ideal gas law. The three gas laws in combination with avogadro s law can be generalized by the ideal gas law. Boyle showed that the volume of a sample of a gas is inversely proportional to its pressure boyle s law charles and gay lussac demonstrated that the volume of a gas is directly proportional to its temperature in kelvins at constant pressure charles s law and avogadro postulated that the volume of a gas is directly proportional to the number of moles of gas present avogadro s law. You can express this mathematically as. This relationship is known as charles s law.
 Source: chem.fsu.edu
Source: chem.fsu.edu
The three gas laws in combination with avogadro s law can be generalized by the ideal gas law. If you increase the pressure 10 times the volume will decrease 10 times. Boyle showed that the volume of a sample of a gas is inversely proportional to its pressure boyle s law charles and gay lussac demonstrated that the volume of a gas is directly proportional to its temperature in kelvins at constant pressure charles s law and avogadro postulated that the volume of a gas is directly proportional to the number of moles of gas present avogadro s law. That means that for example if you double the pressure you will halve the volume. The two terms involved in charles law are directly proportional to each other while the terms involved in boyle s law are inversely proportional.
 Source: youtube.com
Source: youtube.com
This commonly involves explaining how the lung volume may be increased or decreased and thereby cause a relatively lower or higher air pressure within them in keeping with boyle s law. What is the difference between charles law and boyle s law. Boyle showed that the volume of a sample of a gas is inversely proportional to its pressure boyle s law charles and gay lussac demonstrated that the volume of a gas is directly proportional to its temperature in kelvins at constant pressure charles s law and avogadro postulated that the volume of a gas is directly proportional to the number of moles of gas present avogadro s law. You can express this mathematically as. That means that for example if you double the pressure you will halve the volume.
 Source: pinterest.com
Source: pinterest.com
If the absolute temperature of the gas increases the gas volume also increases on the contrary if the absolute temperature of the gas decreases the gas volume also decreases. The two terms involved in charles law are directly proportional to each other while the terms involved in boyle s law are inversely proportional. V α t p constant. Boyle s law is often used as part of an explanation on how the breathing system works in the human body. If the absolute temperature of the gas increases the gas volume also increases on the contrary if the absolute temperature of the gas decreases the gas volume also decreases.
 Source: britannica.com
Source: britannica.com
Boyle s law is often used as part of an explanation on how the breathing system works in the human body. Volume α temperature constant pressure. V α t p constant. This relationship is known as charles s law. Boyle s law charles s law and gay lussac s law form the combined gas law.
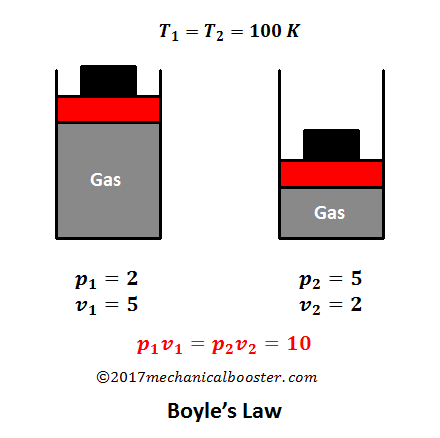 Source: mechanicalbooster.com
Source: mechanicalbooster.com
For a fixed mass of gas at constant temperature the volume is inversely proportional to the pressure. Boyle s law is often used as part of an explanation on how the breathing system works in the human body. If you increase the pressure 10 times the volume will decrease 10 times. This relationship is known as charles s law. The two terms involved in charles law are directly proportional to each other while the terms involved in boyle s law are inversely proportional.
 Source: yumpu.com
Source: yumpu.com
V α t p constant. The three gas laws in combination with avogadro s law can be generalized by the ideal gas law. If the absolute temperature of the gas increases the gas volume also increases on the contrary if the absolute temperature of the gas decreases the gas volume also decreases. This relationship is known as charles s law. This commonly involves explaining how the lung volume may be increased or decreased and thereby cause a relatively lower or higher air pressure within them in keeping with boyle s law.
 Source: pinterest.com
Source: pinterest.com
What is the difference between charles law and boyle s law. Volume α temperature constant pressure. V α t p constant. The two terms involved in charles law are directly proportional to each other while the terms involved in boyle s law are inversely proportional. For a fixed mass of gas at constant temperature the volume is inversely proportional to the pressure.
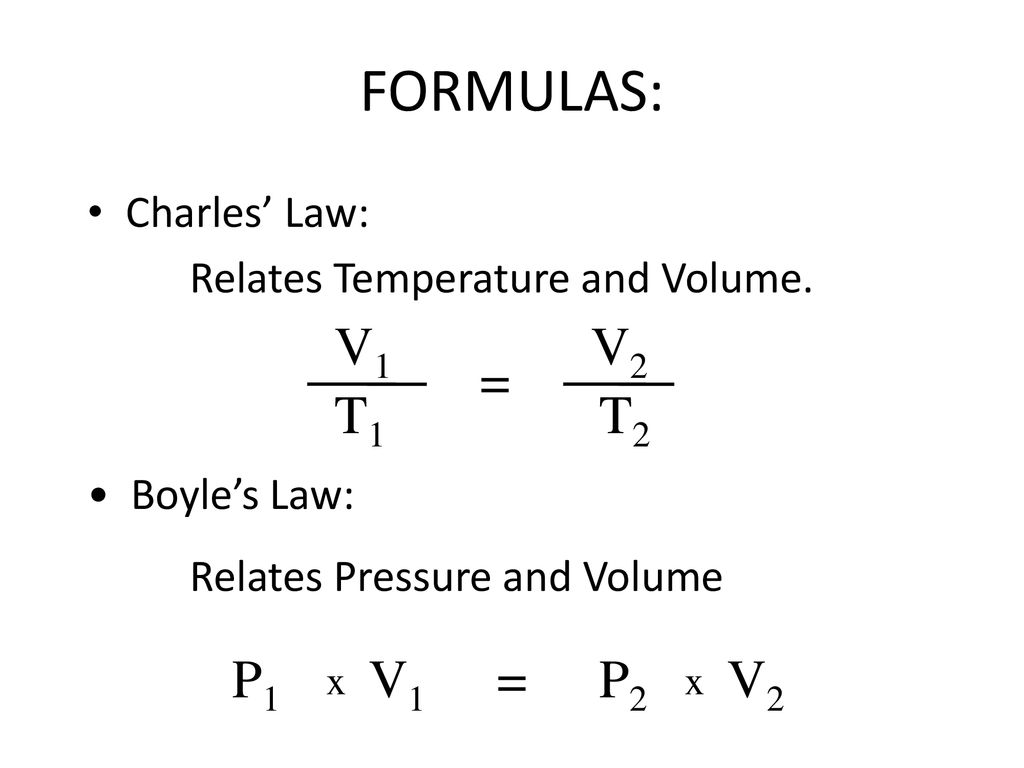 Source: slideplayer.com
Source: slideplayer.com
Boyle showed that the volume of a sample of a gas is inversely proportional to its pressure boyle s law charles and gay lussac demonstrated that the volume of a gas is directly proportional to its temperature in kelvins at constant pressure charles s law and avogadro postulated that the volume of a gas is directly proportional to the number of moles of gas present avogadro s law. The two terms involved in charles law are directly proportional to each other while the terms involved in boyle s law are inversely proportional. V α t p constant. Charles law is defined for a system with a constant pressure while boyle s law is defined for a system with constant temperature. This relationship is known as charles s law.
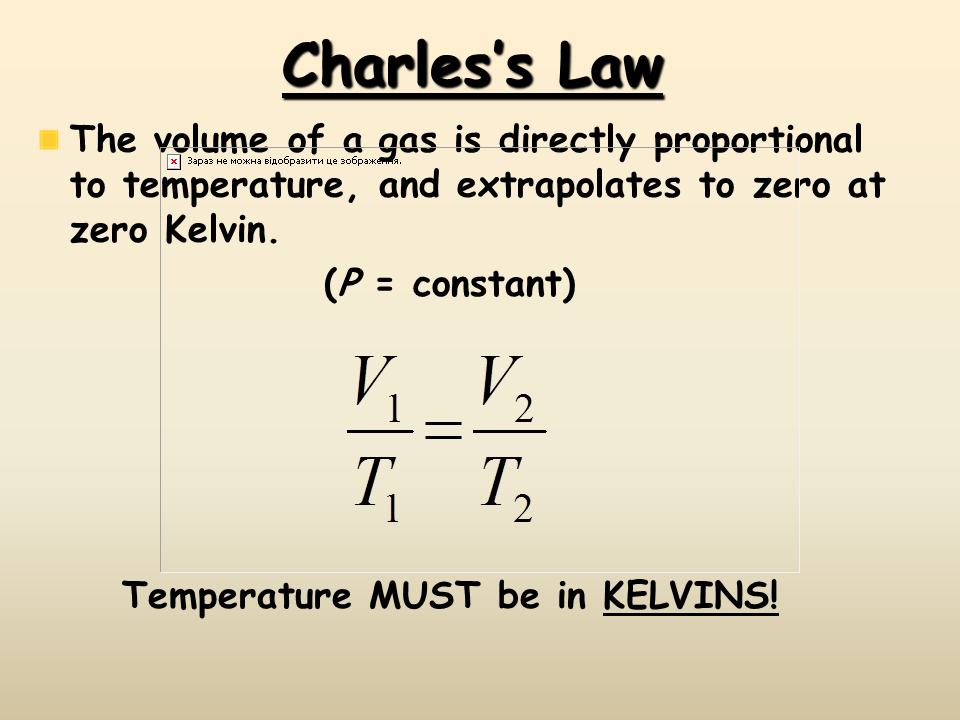
That means that for example if you double the pressure you will halve the volume. What is the difference between charles law and boyle s law. If the absolute temperature of the gas increases the gas volume also increases on the contrary if the absolute temperature of the gas decreases the gas volume also decreases. That means that for example if you double the pressure you will halve the volume. If you increase the pressure 10 times the volume will decrease 10 times.
 Source: tes.com
Source: tes.com
What is the difference between charles law and boyle s law. This relationship is known as charles s law. V α t p constant. If you increase the pressure 10 times the volume will decrease 10 times. For a fixed mass of gas at constant temperature the volume is inversely proportional to the pressure.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title charles and boyles law by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.
