Element vs compound vs mixture
Home » Science Education » Element vs compound vs mixtureElement vs compound vs mixture
Element Vs Compound Vs Mixture. Elements mixtures and compounds. A substance that can be broken down into simpler substances. Key differences between compound and mixture. This picture shows the differences between elements and compounds at an atomic level.
 Elements Compounds And Mixtures Ppt Video Online Download From slideplayer.com
Elements Compounds And Mixtures Ppt Video Online Download From slideplayer.com
Elements allude to those substances that can t be split into simpler substances. A pure substance that cannot be broken down into a simpler substance. A substance that can be broken down into simpler substances. Consists of atoms of two or more different elements bound together can be broken down into a simpler type of matter elements by chemical means but not by physical means has properties that are different from its component elements and. The mixture is the physical combination of substances bonded together in any proportion. Mixtures do not have specific properties or melting and boiling points while compounds have.
A blend of 2 or more kinds of matter each of which has its own.
Elements and compounds are both substances. Elements that compose the compound are chemically combined. They differ from mixtures where different substances mix together but not via atomic bonds. Note that a compound. Elements and compounds are both substances. The mixture is the physical combination of substances bonded together in any proportion.
 Source: pinterest.co.uk
Source: pinterest.co.uk
Chemistry is the study of physical matter which is classified in many different ways such as state of matter gas liquid or solid chemical form element mixture or compound chemical structure atoms or molecules etc and so on. Mixtures do not have specific properties or melting and boiling points while compounds have. Find out in this video. Always contains the same ratio of its component atoms. They differ from mixtures where different substances mix together but not via atomic bonds.
 Source: pinterest.com
Source: pinterest.com
Elements have only 1 type of atoms. The compound is the chemical combination of elements bonded together in specific proportion. Elements and compounds are both substances. A pure substance that cannot be broken down into a simpler substance. Each compound is made up of 2 or more elements that are chemically bonded.
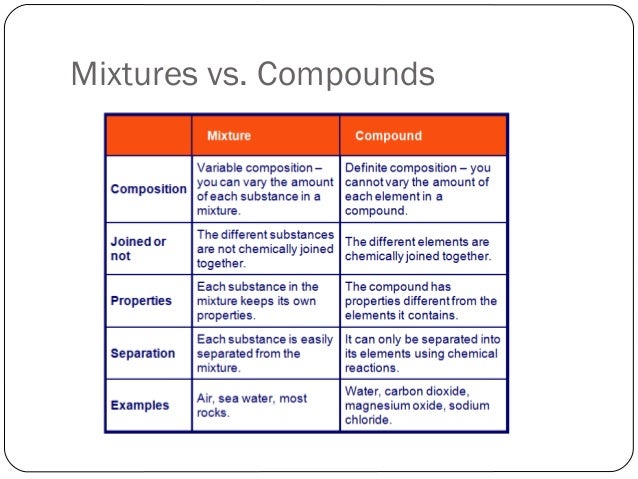 Source: pt.slideshare.net
Source: pt.slideshare.net
Elements have only 1 type of atoms. What is an element mixture and compound learn the basics about what is an element. They contain only one type of molecule. Elements and compounds are both substances. Elements mixtures and compounds.
 Source: diffen.com
Source: diffen.com
Elements and compounds are both substances. A pure substance that cannot be broken down into a simpler substance. The compound is the chemical combination of elements bonded together in specific proportion. Compounds have more than 1. The mixture is the physical combination of substances bonded together in any proportion.
 Source: expii.com
Source: expii.com
It is made up of one type of atom. A blend of 2 or more kinds of matter each of which has its own. Elements allude to those substances that can t be split into simpler substances. How is a mixture done. These compounds are often simply called alloys mixture of metals or a metal with another element.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Find out in this video. They differ from mixtures where different substances mix together but not via atomic bonds. Elements that compose the compound are chemically combined. Elements allude to those substances that can t be split into simpler substances. Elements and compounds are both substances.
 Source: youtube.com
Source: youtube.com
Find out in this video. How is a mixture done. A pure substance that cannot be broken down into a simpler substance. Elements that compose the compound are chemically combined. Chemistry is the study of physical matter which is classified in many different ways such as state of matter gas liquid or solid chemical form element mixture or compound chemical structure atoms or molecules etc and so on.
 Source: diffen.com
Source: diffen.com
The mixture is the physical combination of substances bonded together in any proportion. Mixtures formed due to the physical joining of two or more particles of different elements while compounds form due to chemical bonding between the atoms of two or more elements in a specific ratio. Always contains the same ratio of its component atoms. They differ from mixtures where different substances mix together but not via atomic bonds. A blend of 2 or more kinds of matter each of which has its own.

This picture shows the differences between elements and compounds at an atomic level. Compounds have more than 1. And what is a compound. The mixture is the physical combination of substances bonded together in any proportion. Key differences between compound and mixture.
 Source: proprofs.com
Source: proprofs.com
Note that a compound. Element mixture or compound properties of matter chemistry fuseschoollearn the basics about what an element is how a mixture is done and what a compou. How is a mixture done. What is an element mixture and compound learn the basics about what is an element. Elements that compose the compound are chemically combined.
 Source: slideplayer.com
Source: slideplayer.com
The mixture is the physical combination of substances bonded together in any proportion. Compounds have more than 1. A blend of 2 or more kinds of matter each of which has its own. And what is a compound. Find out in this video.
 Source: pinterest.com
Source: pinterest.com
Chemistry is the study of physical matter which is classified in many different ways such as state of matter gas liquid or solid chemical form element mixture or compound chemical structure atoms or molecules etc and so on. Elements mixtures and compounds. Coordinate bonds a bit more complicated than the other three it is a compound that is a kind of 2 center 2 electron covalent bond and in which the two electrons are derived from the same atom. Note that a compound. Mixtures formed due to the physical joining of two or more particles of different elements while compounds form due to chemical bonding between the atoms of two or more elements in a specific ratio.
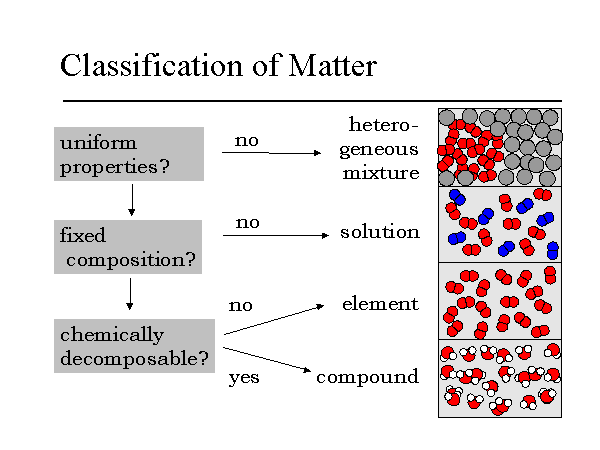 Source: chemsite.lsrhs.net
Source: chemsite.lsrhs.net
They differ from mixtures where different substances mix together but not via atomic bonds. They differ from mixtures where different substances mix together but not via atomic bonds. Each compound is made up of 2 or more elements that are chemically bonded. What is an element mixture and compound learn the basics about what is an element. This picture shows the differences between elements and compounds at an atomic level.

Elements that compose the compound are chemically combined. A pure substance that cannot be broken down into a simpler substance. Mixtures contain different elements and compounds but the ratio is not fixed nor are they combined via chemical bonds. Find out in this video. Consists of atoms of two or more different elements bound together can be broken down into a simpler type of matter elements by chemical means but not by physical means has properties that are different from its component elements and.
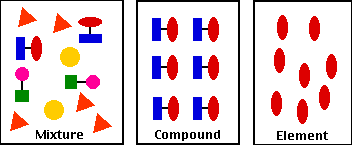 Source: biology-pages.info
Source: biology-pages.info
Key differences between compound and mixture. While the compound is a pure substance the mixture is an impure substance. A pure substance that cannot be broken down into a simpler substance. Coordinate bonds a bit more complicated than the other three it is a compound that is a kind of 2 center 2 electron covalent bond and in which the two electrons are derived from the same atom. Elements and compounds are both substances.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title element vs compound vs mixture by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.
