Is carbon dioxide an element compound or mixture
Home » Science Education » Is carbon dioxide an element compound or mixtureIs carbon dioxide an element compound or mixture
Is Carbon Dioxide An Element Compound Or Mixture. Click to see full answer. Which element do plants release during photosynthesis. Carbon dioxide is a compound because it is comprised of multiple elements carbon and oxygen chemically bonded together. Can the components of a compound or mixture be in any proportion.

Air is not a mixture because of scientists freezing it and finding different liquids it is a mixture because the compounds that make up air e g. Can the components of a compound or mixture be in any proportion. But it has many forms depending on the conditions it was formed at. Carbon dioxide co2 in solid form one carbon atom joined with two oxygen atoms 1 2. Element compound mixture or solution. Compounds elements and mixtures dry ice.
Likewise why is carbon classified as an element rather than a compound.
Can the components of a compound or mixture be in any proportion. Can the components of a compound or mixture be in any proportion. Likewise why is carbon classified as an element rather than a compound. Dry ice solid carbon dioxide. Diamonds are pure carbon but they were formed deep within the earth. Compounds elements and mixtures dry ice.
 Source: youtube.com
Source: youtube.com
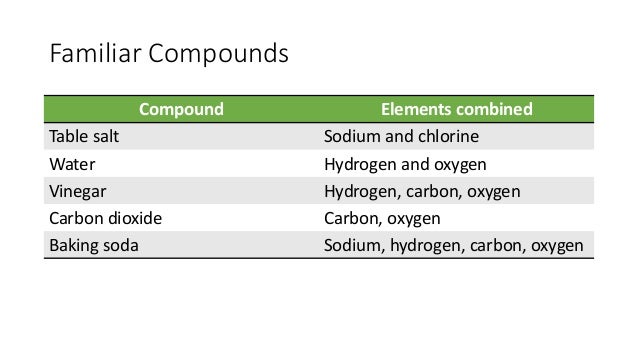
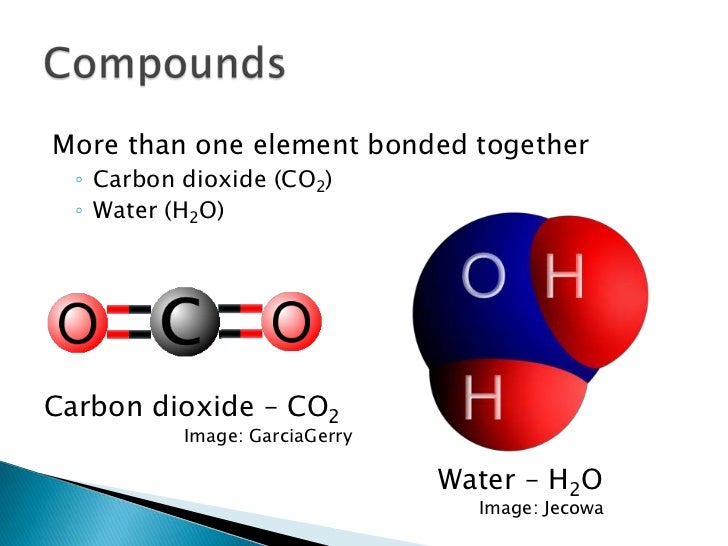
Carbon dioxide co2 in solid form one carbon atom joined with two oxygen atoms 1 2. Element compound mixture or solution. Dry ice is a compound. Carbon dioxide co2 in solid form one carbon atom joined with two oxygen atoms 1 2. Co2 is a compound of one carbon molecule and two molecules of oxygen.
 Source: serpmedia.org
Source: serpmedia.org
Carbon dioxide is a compound because it is comprised of multiple elements carbon and oxygen chemically bonded together. Can the components of a compound or mixture be in any proportion. Dry ice is a compound. Which of the following best describes chicken noodle soup. Air is a mixture of many elements and compounds.
 Source: slideplayer.com
Source: slideplayer.com
Element compound mixture or solution. Learn vocabulary terms and more with flashcards games and other study tools. Can the components of a compound or mixture be in any proportion. Element compound mixture or solution. Compounds elements and mixtures dry ice.
 Source: slideshare.net
Source: slideshare.net
Compounds elements and mixtures dry ice. Likewise why is carbon classified as an element rather than a compound. Diamonds are pure carbon but they were formed deep within the earth. For example consider oil and water they are mixed together but their internal properties remain the same. Mixtures are groups of atoms that include elements and compounds that are not chemically bonded together and may be separated by non chemical physical means.
Source: quora.com
Can the components of a compound or mixture be in any proportion. But it has many forms depending on the conditions it was formed at. Element compound mixture or solution. Air is a mixture of many elements and compounds. Oxygen o2 carbon dioxide co2 and the most important nitrogen which is an element and makes up 78 09 of air are not chemically bound in the way that compounds are.
 Source: slideplayer.com
Source: slideplayer.com
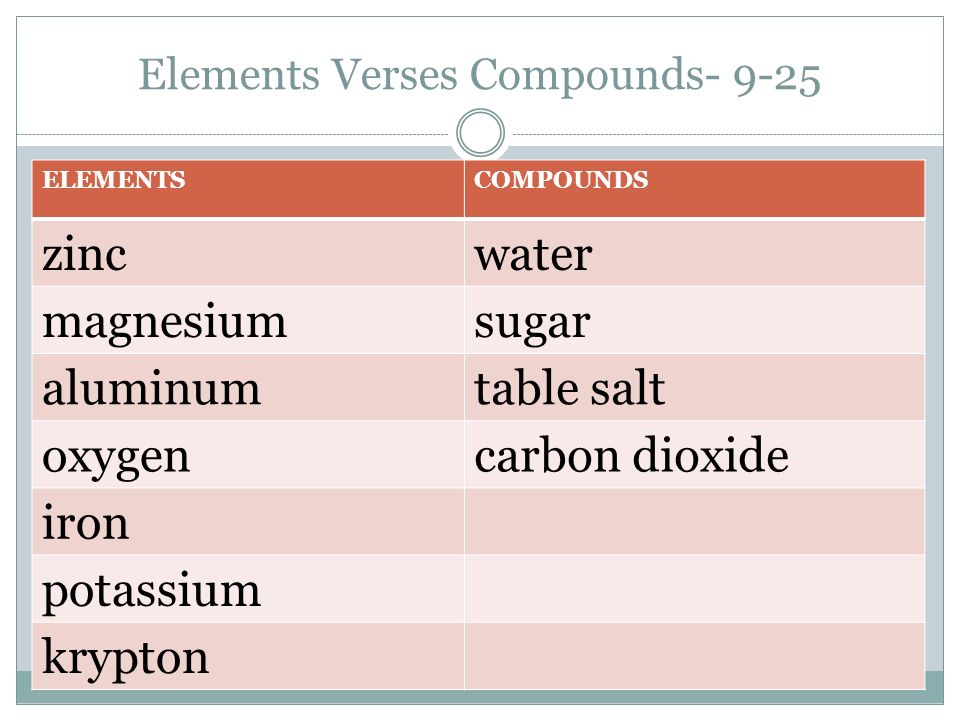
Co2 is a compound of one carbon molecule and two molecules of oxygen. Mixtures are groups of atoms that include elements and compounds that are not chemically bonded together and may be separated by non chemical physical means. Learn vocabulary terms and more with flashcards games and other study tools. Carbon dioxide is a compound because it is comprised of multiple elements carbon and oxygen chemically bonded together. Carbon is an element.
 Source: pt.slideshare.net
Source: pt.slideshare.net
Start studying element compound or mixture. These compounds may not be. For example consider oil and water they are mixed together but their internal properties remain the same. Air is not a mixture because of scientists freezing it and finding different liquids it is a mixture because the compounds that make up air e g. Dry ice solid carbon dioxide.

Oxygen o2 carbon dioxide co2 and the most important nitrogen which is an element and makes up 78 09 of air are not chemically bound in the way that compounds are. Which of the following best describes chicken noodle soup. Mixtures are groups of atoms that include elements and compounds that are not chemically bonded together and may be separated by non chemical physical means. Learn vocabulary terms and more with flashcards games and other study tools. For example consider oil and water they are mixed together but their internal properties remain the same.
 Source: brainly.in
Source: brainly.in
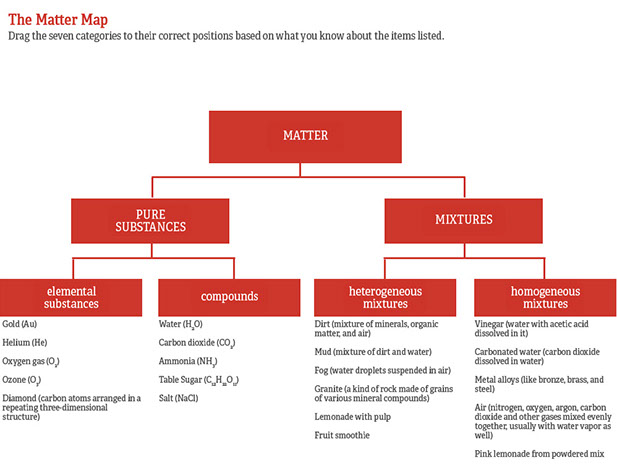
Air is a mixture of many elements and compounds. Learn vocabulary terms and more with flashcards games and other study tools. Air is not a mixture because of scientists freezing it and finding different liquids it is a mixture because the compounds that make up air e g. Is sodium chloride salt an element compound or mixture compound 6 is carbon dioxide an element compound or mixture compound 7 is lead an element compound or mixture element 8 is rock salt an element compound or mixture mixture 9 is sugar solution an element compound or mixture. Carbon is an element.
 Source: brainly.com
Source: brainly.com
But it has many forms depending on the conditions it was formed at. Start studying element compound or mixture. Diamonds are pure carbon but they were formed deep within the earth. Dry ice is a compound. Mixtures are groups of atoms that include elements and compounds that are not chemically bonded together and may be separated by non chemical physical means.
 Source: quora.com
Source: quora.com
Element compound mixture or solution. Which element do plants release during photosynthesis. Air is not a mixture because of scientists freezing it and finding different liquids it is a mixture because the compounds that make up air e g. Compounds elements and mixtures dry ice. Carbon dioxide co2 in solid form one carbon atom joined with two oxygen atoms 1 2.
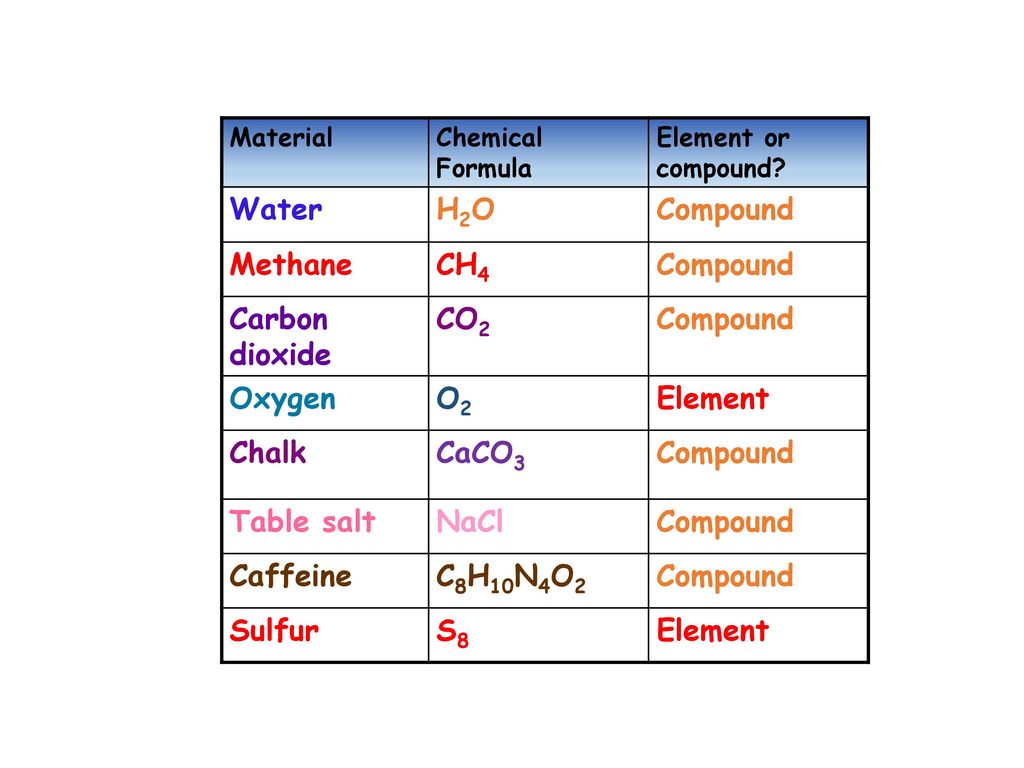 Source: slideplayer.com
Source: slideplayer.com
For example consider oil and water they are mixed together but their internal properties remain the same. Likewise why is carbon classified as an element rather than a compound. Mixtures are groups of atoms that include elements and compounds that are not chemically bonded together and may be separated by non chemical physical means. Learn vocabulary terms and more with flashcards games and other study tools. Carbon dioxide is a compound because it is comprised of multiple elements carbon and oxygen chemically bonded together.
 Source: slideplayer.com
Source: slideplayer.com
Dry ice solid carbon dioxide. Properties are often similar to the properties of its components. Diamonds are pure carbon but they were formed deep within the earth. Likewise why is carbon classified as an element rather than a compound. But it has many forms depending on the conditions it was formed at.
 Source: slideplayer.com
Source: slideplayer.com
Diamonds are pure carbon but they were formed deep within the earth. Air is not a mixture because of scientists freezing it and finding different liquids it is a mixture because the compounds that make up air e g. Can the components of a compound or mixture be in any proportion. Start studying element compound or mixture. Compounds elements and mixtures dry ice.
Source: quora.com
These compounds may not be. Click to see full answer. Element compound mixture or solution. Mixtures are groups of atoms that include elements and compounds that are not chemically bonded together and may be separated by non chemical physical means. Carbon dioxide co2 in solid form one carbon atom joined with two oxygen atoms 1 2.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title is carbon dioxide an element compound or mixture by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.
