Molecular compounds examples
Home » Science Education » Molecular compounds examplesMolecular compounds examples
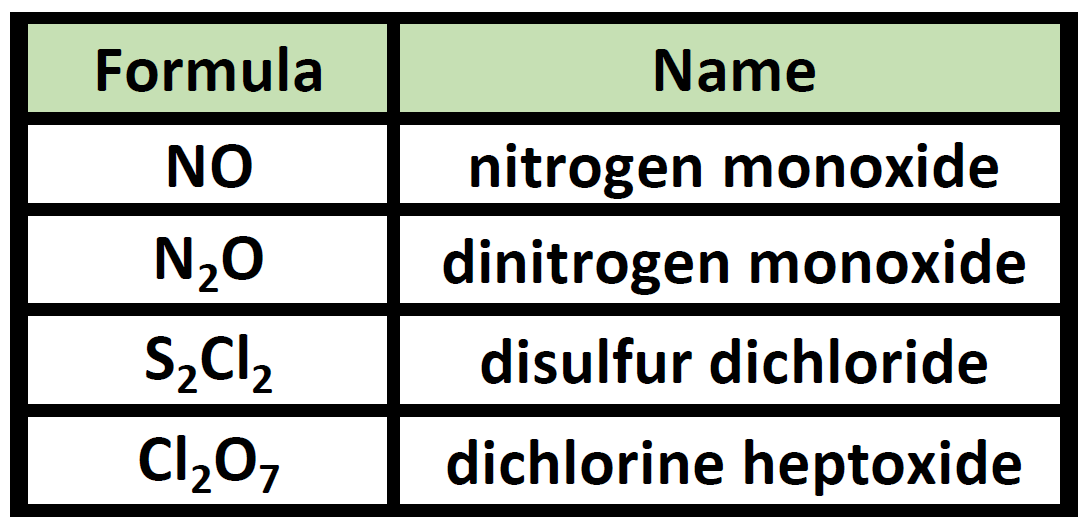
Molecular Compounds Examples. Propose a definition for binary molecular compounds. Examples of covalent compound names so 2 sulfur dioxide sf 6 sulfur hexafluoride ccl 4 carbon tetrachloride ni 3 nitrogen triiodide writing the formula from the name. These compounds are very different from ionic compounds like sodium chloride nacl. Molecular compounds can form compounds with different ratios of their elements so prefixes are used to specify the numbers of atoms of each element in a molecule of the compound.
 Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry From wou.edu
Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry From wou.edu
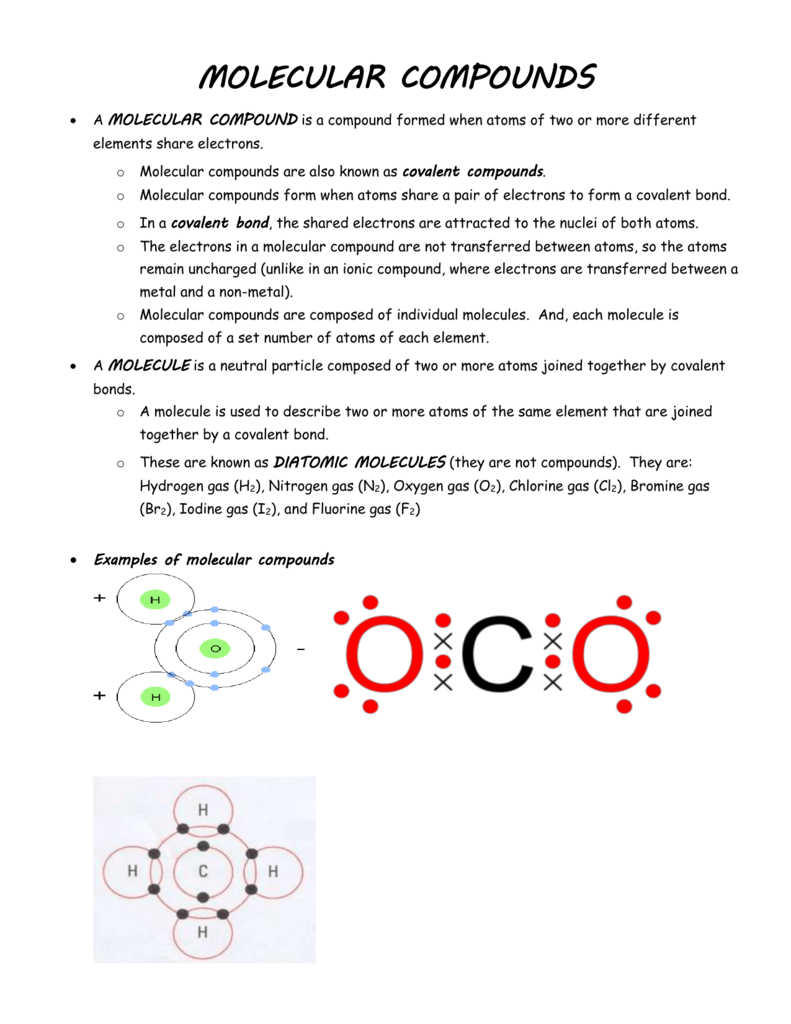
In order to learn the naming convention let s take an example of the common molecular compound water h 2 o. Examples of covalent compound names so 2 sulfur dioxide sf 6 sulfur hexafluoride ccl 4 carbon tetrachloride ni 3 nitrogen triiodide writing the formula from the name. Compounds such as ch oh or pf cl are not binary and compounds such as nacl or cacl are not molecular. Sodium chloride potassium chloride etc. Thus in these compounds covalent bonds are present e g methane ch 4 water h 2 o etc. Examples of molecular compounds with their common names are.
All of the compounds listed in model 2 are binary molecular compounds.
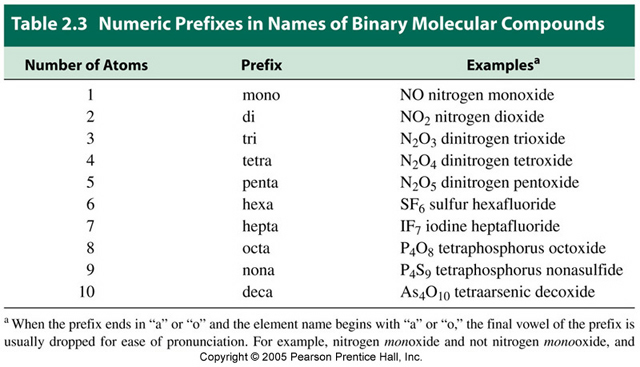
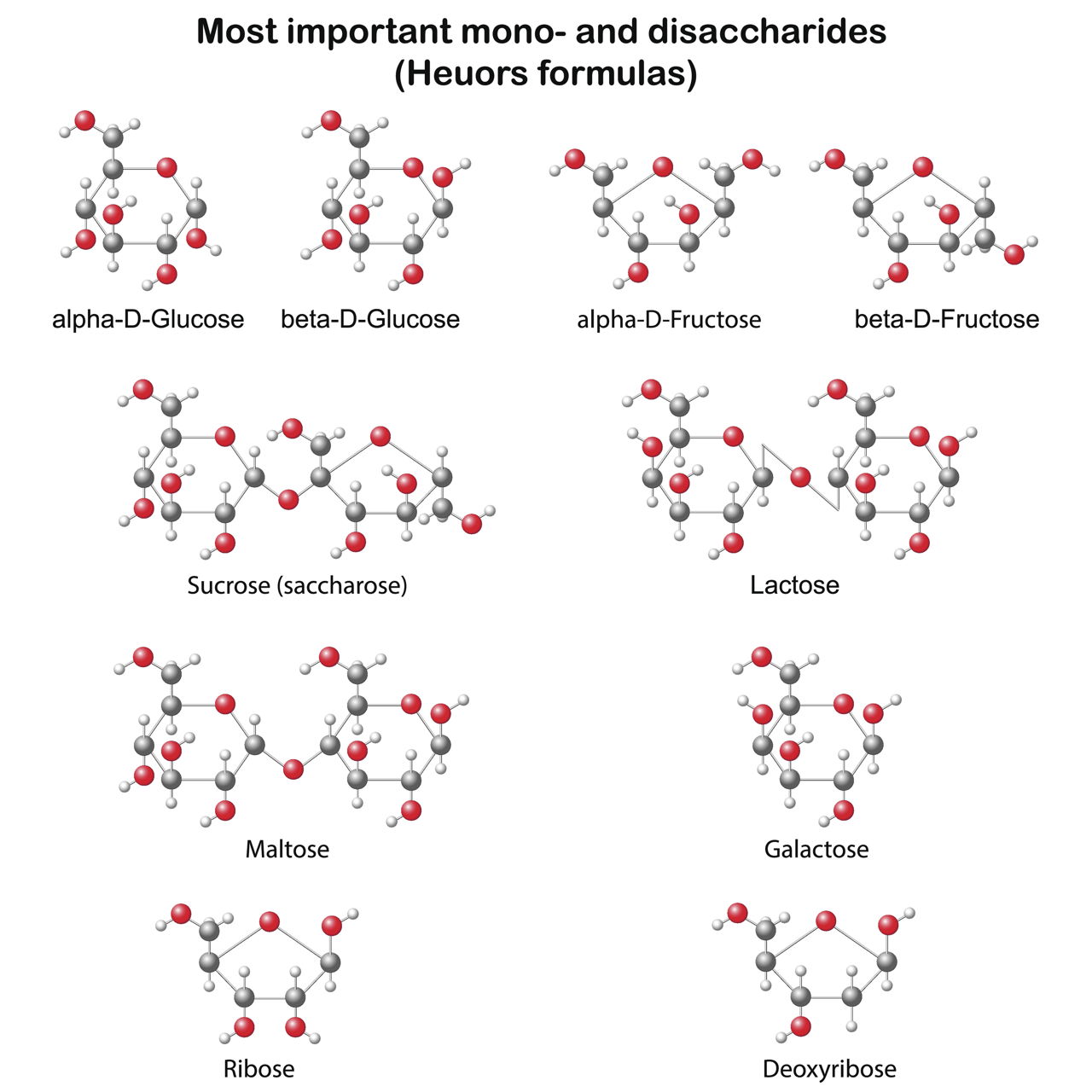
Examples of molecular compounds with their common names are. H 2 o water h 2 o 2 hydrogen peroxide ch 4 methane nh 3 ammonia and so on. Molecular compounds are chemical compounds that take the form of discrete molecules. Molecular compounds are chemical compounds that take the form of discrete molecules. Examples include such familiar substances as water h 2 o and carbon dioxide co 2 figure 3 1. Binary molecular compounds contam two nonmetãllzc elements 15.
 Source: slideplayer.com
Source: slideplayer.com
Compounds such as ch oh or pf cl are not binary and compounds such as nacl or cacl are not molecular. Examples of covalent compound names so 2 sulfur dioxide sf 6 sulfur hexafluoride ccl 4 carbon tetrachloride ni 3 nitrogen triiodide writing the formula from the name. Thus in these compounds covalent bonds are present e g methane ch 4 water h 2 o etc. Molecular compounds are chemical compounds that take the form of discrete molecules. Sodium chloride potassium chloride etc.
 Source: pinterest.com
Source: pinterest.com
These compounds are very different from ionic compounds like sodium chloride nacl. Molecular compounds are chemical compounds that take the form of discrete molecules. Examples include such familiar substances as water h 2 o and carbon dioxide co 2 figure below. These compounds are very different from ionic compounds like sodium chloride nacl. In order to learn the naming convention let s take an example of the common molecular compound water h 2 o.
 Source: wou.edu
Source: wou.edu
All of the compounds listed in model 2 are binary molecular compounds. Molecular compounds are those compounds in which the atoms of the elements share electrons via covalent bonds. Binary molecular compounds contam two nonmetãllzc elements 15. Thus in these compounds covalent bonds are present e g methane ch 4 water h 2 o etc. Examples of molecular compounds with their common names are.
 Source: socratic.org
Source: socratic.org
Propose a definition for binary molecular compounds. Sodium chloride potassium chloride etc. Molecular compounds are chemical compounds that take the form of discrete molecules. H 2 o water h 2 o 2 hydrogen peroxide ch 4 methane nh 3 ammonia and so on. All of the compounds listed in model 2 are binary molecular compounds.
 Source: slideplayer.com
Source: slideplayer.com
Molecular compounds are those compounds in which the atoms of the elements share electrons via covalent bonds. The compounds formed by sharing of electrons between the elements to get inert gas configuration is known as molecular compound. Molecular compounds are chemical compounds that take the form of discrete molecules. These compounds are very different from ionic compounds like sodium chloride nacl. In order to learn the naming convention let s take an example of the common molecular compound water h 2 o.
 Source: bramblechemistry.weebly.com
Source: bramblechemistry.weebly.com
Compounds such as ch oh or pf cl are not binary and compounds such as nacl or cacl are not molecular. Sodium chloride potassium chloride etc. Examples include sf 6 sulfur hexafluoride and n 2 o 4 dinitrogen tetroxide. Examples of covalent compound names so 2 sulfur dioxide sf 6 sulfur hexafluoride ccl 4 carbon tetrachloride ni 3 nitrogen triiodide writing the formula from the name. Examples include such familiar substances as water h 2 o and carbon dioxide co 2 figure 3 1.
 Source: sciencestruck.com
Source: sciencestruck.com
Examples of molecular compounds with their common names are. Examples include sf 6 sulfur hexafluoride and n 2 o 4 dinitrogen tetroxide. Sodium chloride potassium chloride etc. Examples include such familiar substances as water h 2 o and carbon dioxide co 2 figure 3 1. These compounds are very different from ionic compounds like sodium chloride nacl.
 Source: youtube.com
Source: youtube.com
Examples include such familiar substances as water h 2 o and carbon dioxide co 2 figure below. Molecular compounds can form compounds with different ratios of their elements so prefixes are used to specify the numbers of atoms of each element in a molecule of the compound. Examples include sf 6 sulfur hexafluoride and n 2 o 4 dinitrogen tetroxide. These compounds are very different from ionic compounds like sodium chloride nacl. Sodium chloride potassium chloride etc.

Molecular compounds are chemical compounds that take the form of discrete molecules. Molecular compounds are chemical compounds that take the form of discrete molecules. Examples include sf 6 sulfur hexafluoride and n 2 o 4 dinitrogen tetroxide. Thus in these compounds covalent bonds are present e g methane ch 4 water h 2 o etc. Molecular compounds are those compounds in which the atoms of the elements share electrons via covalent bonds.
 Source: studylib.net
Source: studylib.net
Compounds such as ch oh or pf cl are not binary and compounds such as nacl or cacl are not molecular. Compounds such as ch oh or pf cl are not binary and compounds such as nacl or cacl are not molecular. Molecular compounds can form compounds with different ratios of their elements so prefixes are used to specify the numbers of atoms of each element in a molecule of the compound. Molecular compounds are those compounds in which the atoms of the elements share electrons via covalent bonds. Examples of covalent compound names so 2 sulfur dioxide sf 6 sulfur hexafluoride ccl 4 carbon tetrachloride ni 3 nitrogen triiodide writing the formula from the name.
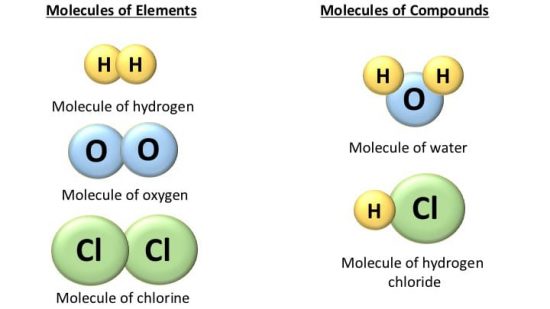 Source: selftution.com
Source: selftution.com
Examples include sf 6 sulfur hexafluoride and n 2 o 4 dinitrogen tetroxide. In order to learn the naming convention let s take an example of the common molecular compound water h 2 o. Molecular compounds are those compounds in which the atoms of the elements share electrons via covalent bonds. Binary molecular compounds contam two nonmetãllzc elements 15. These compounds are very different from ionic compounds like sodium chloride nacl.
 Source: thoughtco.com
Source: thoughtco.com
All of the compounds listed in model 2 are binary molecular compounds. Examples include sf 6 sulfur hexafluoride and n 2 o 4 dinitrogen tetroxide. Molecular compounds can form compounds with different ratios of their elements so prefixes are used to specify the numbers of atoms of each element in a molecule of the compound. These compounds are very different from ionic compounds like sodium chloride nacl. All of the compounds listed in model 2 are binary molecular compounds.
 Source: youtube.com
Source: youtube.com
Thus in these compounds covalent bonds are present e g methane ch 4 water h 2 o etc. In order to learn the naming convention let s take an example of the common molecular compound water h 2 o. The compounds formed by sharing of electrons between the elements to get inert gas configuration is known as molecular compound. Binary molecular compounds contam two nonmetãllzc elements 15. Compounds such as ch oh or pf cl are not binary and compounds such as nacl or cacl are not molecular.
 Source: wou.edu
Source: wou.edu
Examples include such familiar substances as water h 2 o and carbon dioxide co 2 figure 3 1. Propose a definition for binary molecular compounds. Thus in these compounds covalent bonds are present e g methane ch 4 water h 2 o etc. In order to learn the naming convention let s take an example of the common molecular compound water h 2 o. Sodium chloride potassium chloride etc.
 Source: youtube.com
Source: youtube.com
Examples include sf 6 sulfur hexafluoride and n 2 o 4 dinitrogen tetroxide. In order to learn the naming convention let s take an example of the common molecular compound water h 2 o. Examples include such familiar substances as water h 2 o and carbon dioxide co 2 figure 3 1. Compounds such as ch oh or pf cl are not binary and compounds such as nacl or cacl are not molecular. Binary molecular compounds contam two nonmetãllzc elements 15.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title molecular compounds examples by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.
