Properties of sodium carbonate
Home » Science Education » Properties of sodium carbonateProperties of sodium carbonate
Properties Of Sodium Carbonate. At room temperature it is a solid. Observations about the appearance of sodium bicarbonate such as color odor taste and state of matter are all physical properties. Historically it was extracted from the ashes of plants growing in sodium rich soils. It occurs worldwide naturally and specifically in the minerals trona and nahcolite.
 Major Industrial Applications Of Sodium Carbonate From slideshare.net
Major Industrial Applications Of Sodium Carbonate From slideshare.net
There are two forms of sodium carbonate available light soda and dense soda. It occurs worldwide naturally and specifically in the minerals trona and nahcolite. Deposit of sodium carbonate called trona which is contain about 90 percent of mixed carbonate hydrogen carbonate with formula na2co3 nahco3 2h2o. It is odorless and tasteless. Properties of sodium carbonate. Calcium carbonate precipitates by heating sodium carbonate solution with slaked lime.
Historically it was extracted from the ashes of plants growing in sodium rich soils.
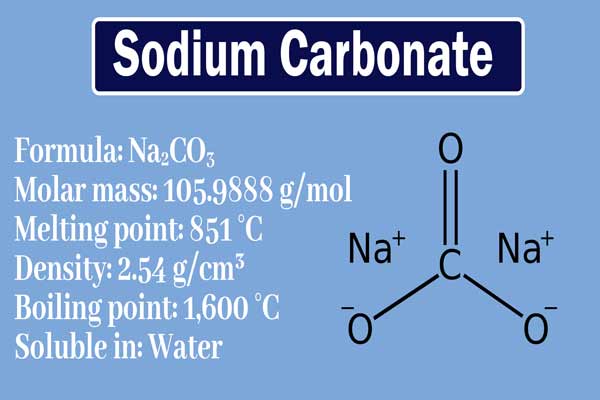
On heating sodium decahydrate loses the water of crystallization to form monohydrate na 2 co 3 h 2 o. There are two forms of sodium carbonate available light soda and dense soda. All forms are white water soluble salts that yield moderately alkaline solutions in water. Its molecular weight is 105 99 g mol. Calcium carbonate precipitates by heating sodium carbonate solution with slaked lime. Sodium carbonate is an important commercial chemical.
 Source: venngage.net
Source: venngage.net
Carbonate salts of those metals are obtained by adding sodium carbonate to aqueous solutions of the salts of most metals. All forms are white water soluble salts that yield moderately alkaline solutions in water. It occurs worldwide naturally and specifically in the minerals trona and nahcolite. It is white crystalline solid which is soluble in water. At room temperature it is a solid.
 Source: slideshare.net
Source: slideshare.net
Sodium carbonate na2co3 is the inorganic compound with the formula na2co3 and its various hydrates. It is also known as washing soda or soda ash. Sodium carbonate is a white crystalline and hygroscopic powder with a purity of 98. This substance also called sodium sesquicarbonate. It occurs worldwide naturally and specifically in the minerals trona and nahcolite.
 Source: slideshare.net
Source: slideshare.net
Sodium carbonate is an important commercial chemical. Sodium carbonate properties precipitation of metallic carbonates. It is odorless and has a bitter salty taste. Sodium bicarbonate is a white crystalline powder that sometimes forms lumps. Its boiling point is 1600.
 Source: en.wikipedia.org
Source: en.wikipedia.org
There are two forms of sodium carbonate available light soda and dense soda. Its boiling point is 1600. Properties of sodium carbonate. Sodium bicarbonate is a white crystalline powder that sometimes forms lumps. It is generally known as washing soda.

This substance also called sodium sesquicarbonate. It is soluble in water. Properties of sodium carbonate. When it is added to water it breaks down into carbonic acid and sodium hydroxide lye. Properties of sodium carbonate.
 Source: chemistrypage.in
Source: chemistrypage.in
Sodium carbonate is an important commercial chemical. Sodium carbonate is white crystalline solid exists in nature as a decahydrate a 2 co 3 10h 2 o. This substance also called sodium sesquicarbonate. Properties of sodium carbonate. Sodium carbonate is readily soluble in water.
 Source: slideshare.net
Source: slideshare.net
Properties of sodium carbonate physical properties. Its melting point is 851. Properties of sodium carbonate. It is soluble in water. Sodium carbonate na2co3 is the inorganic compound with the formula na2co3 and its various hydrates.
 Source: youtube.com
Source: youtube.com
Sodium sesquicarbonate is not a mixture of two compound but it is a single compound with actual formula na2 hco3 co3 2h2o. There are two forms of sodium carbonate available light soda and dense soda. It is white crystalline solid which is soluble in water. Sodium carbonate is a white crystalline and hygroscopic powder with a purity of 98. Sodium carbonate properties precipitation of metallic carbonates.
 Source: researchgate.net
Source: researchgate.net
Sodium sesquicarbonate is not a mixture of two compound but it is a single compound with actual formula na2 hco3 co3 2h2o. Properties of sodium carbonate. It is soluble in water. Historically it was extracted from the ashes of plants growing in sodium rich soils. When it is added to water it breaks down into carbonic acid and sodium hydroxide lye.

Carbonate salts of those metals are obtained by adding sodium carbonate to aqueous solutions of the salts of most metals. Reaction with dilute hcl na 2 co 3 2hcl dil. It is odorless and has a bitter salty taste. The action of heat on sodium carbonate. Its boiling point is 1600.
 Source: youtube.com
Source: youtube.com
Its boiling point is 1600. Sodium carbonate is a white crystalline powder or grayish white lumps. Sodium carbonate is white crystalline solid exists in nature as a decahydrate a 2 co 3 10h 2 o. Observations about the appearance of sodium bicarbonate such as color odor taste and state of matter are all physical properties. Calcium carbonate precipitates by heating sodium carbonate solution with slaked lime.
 Source: scienceeasylearning.wordpress.com
Source: scienceeasylearning.wordpress.com
On heating sodium decahydrate loses the water of crystallization to form monohydrate na 2 co 3 h 2 o. The action of heat on sodium carbonate. Its density is 2 54 g cm 3. At room temperature it is a solid. This substance also called sodium sesquicarbonate.
 Source: britannica.com
Source: britannica.com
Carbonate salts of those metals are obtained by adding sodium carbonate to aqueous solutions of the salts of most metals. At room temperature sodium carbonate na 2 co 3 is an odorless grayish white powder which is hygroscopic. Another familiar compound that has this hygroscopic quality is sugar. The action of heat on sodium carbonate. Its melting point is 851.
 Source: assignmentpoint.com
Source: assignmentpoint.com
All forms are white water soluble salts that yield moderately alkaline solutions in water. It is odorless and tasteless. 2nacl h 2 o co 2. This substance also called sodium sesquicarbonate. Impurities of sodium carbonate may include water 1 5 sodium chloride 0 5 sulphate 0 1 calcium 0 1 magnesium 0 1 and iron 0 004.
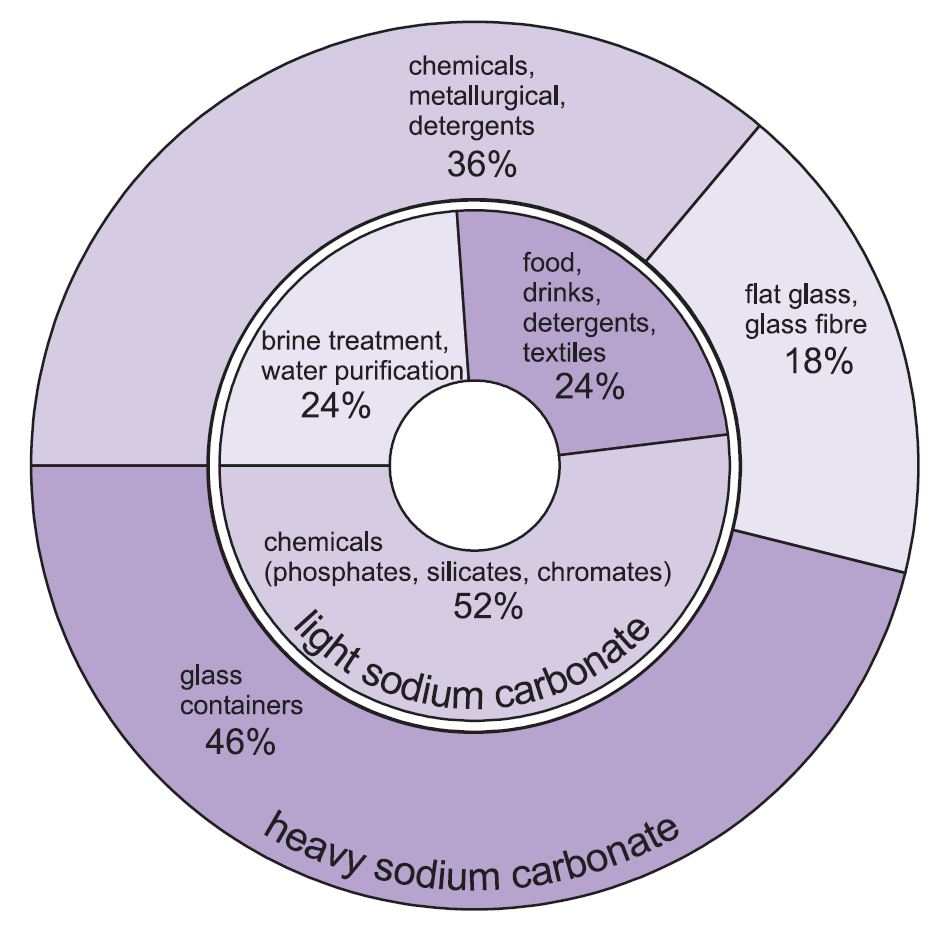
Sodium bicarbonate is a white crystalline powder that sometimes forms lumps. Its boiling point is 1600. Sodium sesquicarbonate is not a mixture of two compound but it is a single compound with actual formula na2 hco3 co3 2h2o. Sodium carbonate is alkali with a high ph when in concentrated solutions. Calcium carbonate precipitates by heating sodium carbonate solution with slaked lime.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title properties of sodium carbonate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.
