Sodium carbonate acid or base
Home » Science Education » Sodium carbonate acid or baseSodium carbonate acid or base
Sodium Carbonate Acid Or Base. Sodium carbonate na 2 co 3 also known as washing soda soda ash and soda crystals and in the monohydrate form as crystal carbonate is the water soluble sodium salt of carbonic acid. Wiki user answered. Historically it was extracted from the ashes of plants growing in sodium rich soils. All forms are white water soluble salts that yield moderately alkaline solutions in water.
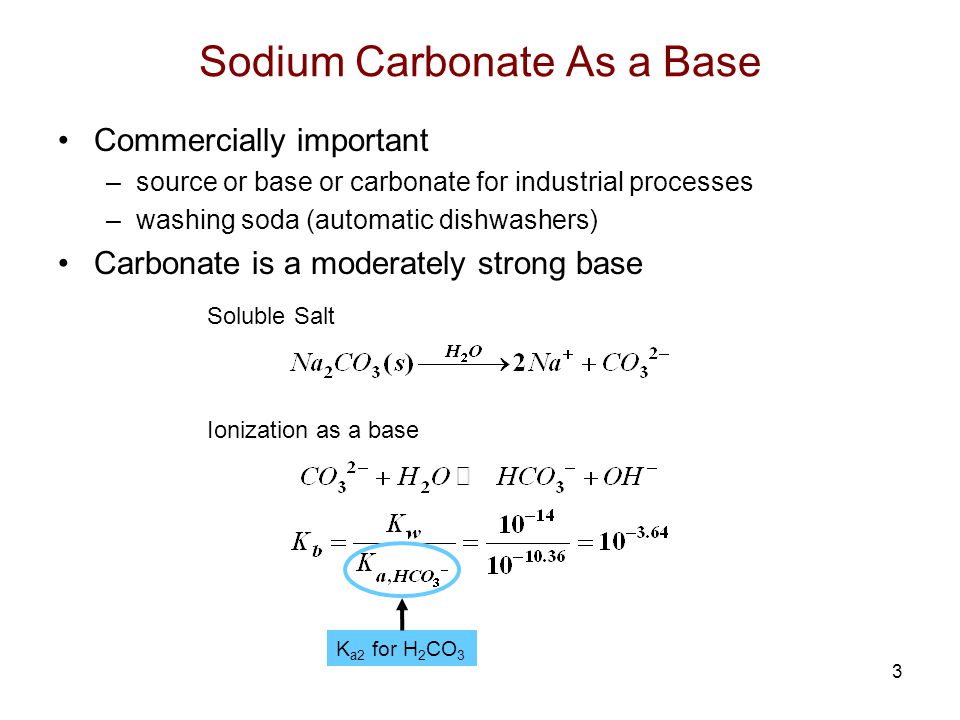 Titration Of Sodium Carbonate Ppt Video Online Download From slideplayer.com
Titration Of Sodium Carbonate Ppt Video Online Download From slideplayer.com
All forms are white water soluble salts that yield moderately alkaline solutions in water. Often found in powder form it s used in a wide range of industries such as in cleaning and personal care products and as a fungicide microbicide herbicide and ph adjuster. It most commonly occurs as a crystalline decahydrate which readily effloresces to form a white powder the monohydrate. Sodium carbonate acid or base. Acid name 1 mm 10 mm. Sodium carbonate na 2 co 3 also known as washing soda soda ash and soda crystals and in the monohydrate form as crystal carbonate is the water soluble sodium salt of carbonic acid.
It most commonly occurs as a crystalline decahydrate which readily effloresces to form a white powder the monohydrate.
Historically it was extracted from the ashes of plants growing in sodium rich soils. Sodium carbonate na 2 co 3 also known as washing soda soda ash and soda crystals and in the monohydrate form as crystal carbonate is the water soluble sodium salt of carbonic acid. Acid name 1 mm 10 mm. Ph of common acids and bases. It most commonly occurs as a crystalline decahydrate which readily effloresces to form a white powder the monohydrate. Calculated ph values of common acids and bases for 1 10 and 100 mmol l valid for standard conditions at 25 c.
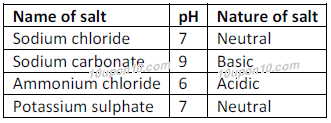 Source: 10upon10.com
Source: 10upon10.com
Asked by wiki user. Because the ashes of these sodium rich plants were noticeably different from ashes of wood sodium carbonate became known as soda ash it is produced in large quantities from sodium chloride and limestone by the solvay. D sodium carbonate na 2 co 3 is the salt formed by the combination of a weak acid such as carbonic acid h 2 co 3 and a strong base such as sodium hydroxide naoh. Asked by wiki user. Soda ash also known as sodium carbonate na2co3 is an alkali chemical refined from the mineral trona or naturally occurring sodium carbonate bearing brines the soda ash from both is referred to as natural soda ash or manufactured from one of several chemical processes the soda ash from this process is referred to as synthetic soda ash.
 Source: goodscience.com.au
Source: goodscience.com.au
Often found in powder form it s used in a wide range of industries such as in cleaning and personal care products and as a fungicide microbicide herbicide and ph adjuster. Often found in powder form it s used in a wide range of industries such as in cleaning and personal care products and as a fungicide microbicide herbicide and ph adjuster. Sodium carbonate na2co3 is a moderately strong base used commonly in organic chemistry. 0 0 1. Wiki user answered.
 Source: sites.google.com
Source: sites.google.com
Because the ashes of these sodium rich plants were noticeably different from ashes of wood sodium carbonate became known as soda ash it is produced in large quantities from sodium chloride and limestone by the solvay. Soda ash also known as sodium carbonate na2co3 is an alkali chemical refined from the mineral trona or naturally occurring sodium carbonate bearing brines the soda ash from both is referred to as natural soda ash or manufactured from one of several chemical processes the soda ash from this process is referred to as synthetic soda ash. Sodium carbonate acid or base. 0 0 1. It most commonly occurs as a crystalline decahydrate which readily effloresces to form a white powder the monohydrate.

Asked by wiki user. Calculated ph values of common acids and bases for 1 10 and 100 mmol l valid for standard conditions at 25 c. Because the ashes of these sodium rich plants were noticeably different from ashes of wood sodium carbonate became known as soda ash it is produced in large quantities from sodium chloride and limestone by the solvay. 0 0 1. All forms are white water soluble salts that yield moderately alkaline solutions in water.
 Source: meritnation.com
Source: meritnation.com
It is regularly employed as the base of choice for palladium catalyzed reactions such as suzuki couplings. Sodium carbonate na2co3 is the inorganic compound with the formula na2co3 and its various hydrates. Ph of common acids and bases. It is regularly employed as the base of choice for palladium catalyzed reactions such as suzuki couplings. It most commonly occurs as a crystalline decahydrate which readily effloresces to form a white powder the monohydrate.
 Source: youtube.com
Source: youtube.com
All forms are white water soluble salts that yield moderately alkaline solutions in water. Sodium carbonate na 2 co 3 also known as washing soda soda ash and soda crystals and in the monohydrate form as crystal carbonate is the water soluble sodium salt of carbonic acid. Ph of common acids and bases. Sodium carbonate na2co3 is a moderately strong base used commonly in organic chemistry. Sodium carbonate soda ash 10 52.
 Source: chegg.com
Source: chegg.com
It most commonly occurs as a crystalline decahydrate which readily effloresces to form a white powder the monohydrate. It is regularly employed as the base of choice for palladium catalyzed reactions such as suzuki couplings. It most commonly occurs as a crystalline decahydrate which readily effloresces to form a white powder the monohydrate. Sodium carbonate na2co3 is a moderately strong base used commonly in organic chemistry. Wiki user answered.
 Source: youtube.com
Source: youtube.com
Sodium carbonate acid or base. D sodium carbonate na 2 co 3 is the salt formed by the combination of a weak acid such as carbonic acid h 2 co 3 and a strong base such as sodium hydroxide naoh. Asked by wiki user. Soda ash also known as sodium carbonate na2co3 is an alkali chemical refined from the mineral trona or naturally occurring sodium carbonate bearing brines the soda ash from both is referred to as natural soda ash or manufactured from one of several chemical processes the soda ash from this process is referred to as synthetic soda ash. Sodium carbonate na2co3 is the inorganic compound with the formula na2co3 and its various hydrates.
 Source: slideplayer.com
Source: slideplayer.com
Wiki user answered. All forms are white water soluble salts that yield moderately alkaline solutions in water. Wiki user answered. Often found in powder form it s used in a wide range of industries such as in cleaning and personal care products and as a fungicide microbicide herbicide and ph adjuster. Sodium carbonate is a strong alkali base used in green cleaning products.
 Source: youtube.com
Source: youtube.com
Sodium carbonate is also used for other reactions that require a base to neutralize acids that form during reactions. D sodium carbonate na 2 co 3 is the salt formed by the combination of a weak acid such as carbonic acid h 2 co 3 and a strong base such as sodium hydroxide naoh. Wiki user answered. Sodium carbonate is a strong alkali base used in green cleaning products. Sodium carbonate na2co3 is the inorganic compound with the formula na2co3 and its various hydrates.
 Source: youtube.com
Source: youtube.com
Calculated ph values of common acids and bases for 1 10 and 100 mmol l valid for standard conditions at 25 c. Calculated ph values of common acids and bases for 1 10 and 100 mmol l valid for standard conditions at 25 c. Ph of common acids and bases. It most commonly occurs as a crystalline decahydrate which readily effloresces to form a white powder the monohydrate. Historically it was extracted from the ashes of plants growing in sodium rich soils.
 Source: slideplayer.com
Source: slideplayer.com
Often found in powder form it s used in a wide range of industries such as in cleaning and personal care products and as a fungicide microbicide herbicide and ph adjuster. Sodium carbonate na2co3 is the inorganic compound with the formula na2co3 and its various hydrates. Sodium carbonate soda ash 10 52. All forms are white water soluble salts that yield moderately alkaline solutions in water. Sodium carbonate na 2 co 3 also known as washing soda soda ash and soda crystals and in the monohydrate form as crystal carbonate is the water soluble sodium salt of carbonic acid.
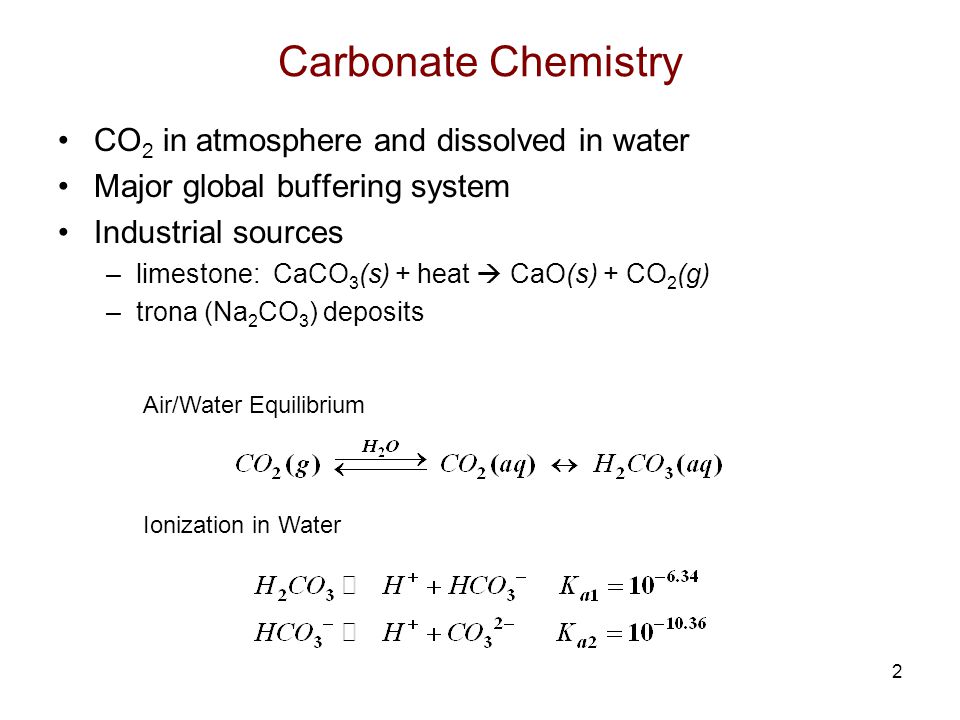 Source: slideplayer.com
Source: slideplayer.com
Calculated ph values of common acids and bases for 1 10 and 100 mmol l valid for standard conditions at 25 c. It is regularly employed as the base of choice for palladium catalyzed reactions such as suzuki couplings. Sodium carbonate is a strong alkali base used in green cleaning products. Wiki user answered. Sodium carbonate na2co3 is the inorganic compound with the formula na2co3 and its various hydrates.
 Source: slideplayer.com
Source: slideplayer.com
Soda ash also known as sodium carbonate na2co3 is an alkali chemical refined from the mineral trona or naturally occurring sodium carbonate bearing brines the soda ash from both is referred to as natural soda ash or manufactured from one of several chemical processes the soda ash from this process is referred to as synthetic soda ash. Sodium carbonate acid or base. D sodium carbonate na 2 co 3 is the salt formed by the combination of a weak acid such as carbonic acid h 2 co 3 and a strong base such as sodium hydroxide naoh. Sodium carbonate is also used for other reactions that require a base to neutralize acids that form during reactions. Often found in powder form it s used in a wide range of industries such as in cleaning and personal care products and as a fungicide microbicide herbicide and ph adjuster.
 Source: youtube.com
Source: youtube.com
Acid name 1 mm 10 mm. Sodium carbonate na2co3 is the inorganic compound with the formula na2co3 and its various hydrates. D sodium carbonate na 2 co 3 is the salt formed by the combination of a weak acid such as carbonic acid h 2 co 3 and a strong base such as sodium hydroxide naoh. 0 0 1. Soda ash also known as sodium carbonate na2co3 is an alkali chemical refined from the mineral trona or naturally occurring sodium carbonate bearing brines the soda ash from both is referred to as natural soda ash or manufactured from one of several chemical processes the soda ash from this process is referred to as synthetic soda ash.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title sodium carbonate acid or base by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.
