Starch iodine reaction
Home » Science Education » Starch iodine reactionStarch iodine reaction
Starch Iodine Reaction. Iodine is not very soluble in water therefore the iodine reagent is made by dissolving iodine in water in the presence of potassium iodide. The starch iodine reaction by n. Soluble starch solution is added. 1924 28 2 125 130.
 Iodine Starch Test Wikipedia From en.wikipedia.org
Iodine Starch Test Wikipedia From en.wikipedia.org
1924 28 2 125 130. The interaction between starch and the triiodide anion i 3 is the basis for iodometry. Low temperature phase transformation accompanied with charge transfer reaction of polyiodide ions encapsulated in single walled carbon nanotubes. Using iodine to test for the presence of starch is a common experiment. The combination of starch and iodine is intensely blue black. The starch iodine test will help the physician determine where on the skin persistent excessive sweating is occurring.
It was discovered by hans heinrich landolt in 1886.
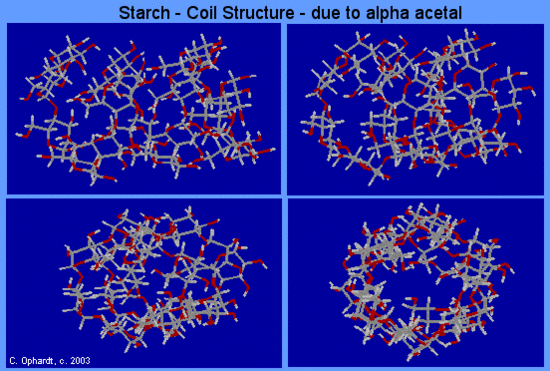
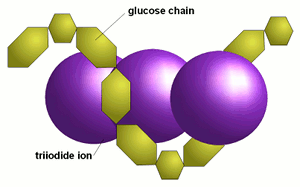
Soluble starch solution is added. Only iodine element in the presence of iodide ion will give the characteristic blue black color. Starch in ter acts with io dine form ing in clu sion com pounds which are called clathrates. Low temperature phase transformation accompanied with charge transfer reaction of polyiodide ions encapsulated in single walled carbon nanotubes. Soluble starch solution is added. The starch iodine reaction by n.

The starch iodine reaction by n. When following the changes in some inorganic oxidation reduction reactions iodine may be used as an indicator to follow the changes of iodide ion and iodine element. The shade of the starch complex is deep to the point that it can be recognized outwardly when the grouping of the iodine is as low as 0 00002 m at 20 c. Dhar two distinct views have been put forward in order to explain the mecha nism of the starch iodine reaction. Iodine ki reagent.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Using iodine to test for the presence of starch is a common experiment. The iodine clock reaction is a classical chemical clock demonstration experiment to display chemical kinetics in action. Granules of wheat starch stained with iodine photographed through a light microscope wikimedia in this ar ti cle we will use a 5 al co hol io dine so lu tion which is used in medicine. After a short time delay the liquid suddenly turns to a shade of dark blue due to the formation of a triiodide s. Two colourless solutions are mixed and at first there is no visible reaction.
 Source: researchgate.net
Source: researchgate.net
A solution of iodine i 2 and potassium iodide ki in water has a light orange brown color. Follow proper patient preparation reconstitution and administration protocol to administer a few units approximately 2 5 u per site of onabotulinumtoxina to the affected areas. After a short time delay the liquid suddenly turns to a shade of dark blue due to the formation of a triiodide s. Amylose in starch is responsible for the formation of a deep blue color in the presence of iodine. Starch shapes an extremely dull blue dark complex with tri iodide which can be made by blending iodine with iodide regularly from potassium iodide.
 Source: researchgate.net
Source: researchgate.net
Dhar two distinct views have been put forward in order to explain the mecha nism of the starch iodine reaction. Iodine ki reagent. Only iodine element in the presence of iodide ion will give the characteristic blue black color. Granules of wheat starch stained with iodine photographed through a light microscope wikimedia in this ar ti cle we will use a 5 al co hol io dine so lu tion which is used in medicine. Soluble starch solution is added.
 Source: slideshare.net
Source: slideshare.net
Iodine ki reagent. Dhar two distinct views have been put forward in order to explain the mecha nism of the starch iodine reaction. The starch iodine test will help the physician determine where on the skin persistent excessive sweating is occurring. The journal of physical chemistry c. Amylose in starch is responsible for the formation of a deep blue color in the presence of iodine.
 Source: brilliantbiologystudent.weebly.com
Source: brilliantbiologystudent.weebly.com
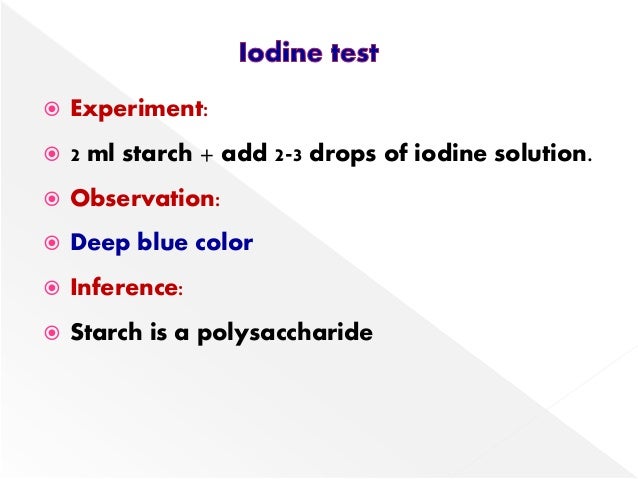
Soluble starch solution is added. The combination of starch and iodine is intensely blue black. If it is added to a sample that contains starch such as the bread pictured above the color changes to a deep blue. The interaction between starch and the triiodide anion i 3 is the basis for iodometry. The iodine starch test is a chemical reaction that is used to test for the presence of starch or for iodine.
 Source: chemistry.elmhurst.edu
Source: chemistry.elmhurst.edu
The axilla should be re mapped using minor s starch iodine test. Starch shapes an extremely dull blue dark complex with tri iodide which can be made by blending iodine with iodide regularly from potassium iodide. Using iodine to test for the presence of starch is a common experiment. The starch iodine reaction by n. The iodine clock reaction is a classical chemical clock demonstration experiment to display chemical kinetics in action.
 Source: en.wikipedia.org
Source: en.wikipedia.org
A solution of iodine i 2 and potassium iodide ki in water has a light orange brown color. After a short time delay the liquid suddenly turns to a shade of dark blue due to the formation of a triiodide s. The iodine clock reaction is a classical chemical clock demonstration experiment to display chemical kinetics in action. When following the changes in some inorganic oxidation reduction reactions iodine may be used as an indicator to follow the changes of iodide ion and iodine element. Two colourless solutions are mixed and at first there is no visible reaction.
 Source: chegg.com
Source: chegg.com
The starch iodine test will help the physician determine where on the skin persistent excessive sweating is occurring. Soluble starch solution is added. Starch shapes an extremely dull blue dark complex with tri iodide which can be made by blending iodine with iodide regularly from potassium iodide. Using iodine to test for the presence of starch is a common experiment. Follow proper patient preparation reconstitution and administration protocol to administer a few units approximately 2 5 u per site of onabotulinumtoxina to the affected areas.
 Source: braukaiser.com
Source: braukaiser.com
Granules of wheat starch stained with iodine photographed through a light microscope wikimedia in this ar ti cle we will use a 5 al co hol io dine so lu tion which is used in medicine. Two colourless solutions are mixed and at first there is no visible reaction. If it is added to a sample that contains starch such as the bread pictured above the color changes to a deep blue. Iodine is not very soluble in water therefore the iodine reagent is made by dissolving iodine in water in the presence of potassium iodide. Starch in ter acts with io dine form ing in clu sion com pounds which are called clathrates.
 Source: melscience.com
Source: melscience.com
The axilla should be re mapped using minor s starch iodine test. The starch iodine reaction by n. Starch shapes an extremely dull blue dark complex with tri iodide which can be made by blending iodine with iodide regularly from potassium iodide. When following the changes in some inorganic oxidation reduction reactions iodine may be used as an indicator to follow the changes of iodide ion and iodine element. Soluble starch solution is added.
 Source: youtube.com
Source: youtube.com
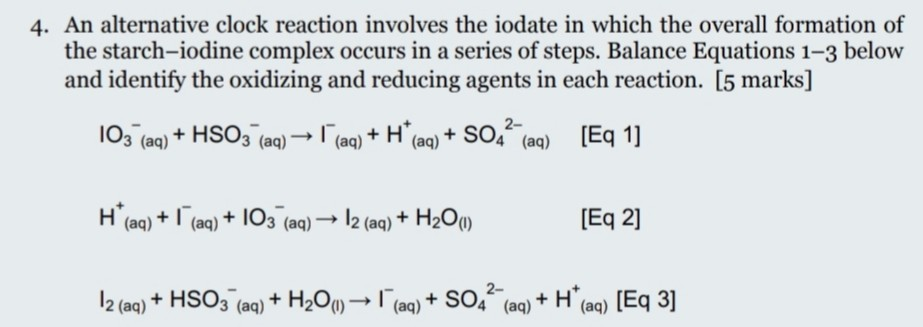
Two colourless solutions are mixed and at first there is no visible reaction. Amylose in starch is responsible for the formation of a deep blue color in the presence of iodine. The combination of starch and iodine is intensely blue black. The iodine clock reaction exists in several variations which each involve iodine species and redox reagents in the presence of starch. Several authors notably rouvierl euler and myrback2 and others have formulated the view that on the addition of iodine to starch paste definite chemical compounde are formed.
 Source: degruyter.com
Source: degruyter.com
The combination of starch and iodine is intensely blue black. When following the changes in some inorganic oxidation reduction reactions iodine may be used as an indicator to follow the changes of iodide ion and iodine element. Using iodine to test for the presence of starch is a common experiment. Dhar two distinct views have been put forward in order to explain the mecha nism of the starch iodine reaction. A solution of iodine i 2 and potassium iodide ki in water has a light orange brown color.
 Source: researchgate.net
Source: researchgate.net
Two colourless solutions are mixed and at first there is no visible reaction. Iodine is not very soluble in water therefore the iodine reagent is made by dissolving iodine in water in the presence of potassium iodide. Follow proper patient preparation reconstitution and administration protocol to administer a few units approximately 2 5 u per site of onabotulinumtoxina to the affected areas. The axilla should be re mapped using minor s starch iodine test. Using iodine to test for the presence of starch is a common experiment.
 Source: chemistryviews.org
Source: chemistryviews.org
Starch in ter acts with io dine form ing in clu sion com pounds which are called clathrates. After a short time delay the liquid suddenly turns to a shade of dark blue due to the formation of a triiodide s. Only iodine element in the presence of iodide ion will give the characteristic blue black color. Neither iodine element alone nor iodide ions alone will give the color result. Iodine is not very soluble in water therefore the iodine reagent is made by dissolving iodine in water in the presence of potassium iodide.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title starch iodine reaction by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.
